Forschung
Hier finden Sie in englischer Sprache Ausführungen zum Bereich Forschung des Adipositas- und Stoffwechselzentrums.

Prof. Dr. med. Wiebke Fenske
Principle Investigator
Enable JavaScript to view protected content.

PD Dr. habil. Andreas Till
Lab Head
Enable JavaScript to view protected content.

Univ. Prof. Dr. Alexander Pfeifer
Principle Investigator
Enable JavaScript to view protected content.

Dr. med. Charlotte Fries
Clinical scientist
Enable JavaScript to view protected content.

Dr. med. Jenny Bischoff
Clinical scientist
Enable JavaScript to view protected content.

Tabea Pöhler, MSc, DDG
Clinical scientist, translation
Enable JavaScript to view protected content.

Alexander Heer
Doctoral Candidate, MD
Enable JavaScript to view protected content.

Cristina Göbbel, MTA
Lab technician
Enable JavaScript to view protected content.

Katharina Brands, MSc
Lab technician
Enable JavaScript to view protected content.

PD Dr. med. Philipp Lingohr
Senior Physician

Dr. med. Azin Jafari
Clinical Scientist

Dr. med. Anna Wöstemeier
Clinical Scientist

Dr. med. Lara Braun
Clinical Scientist
Research Interests
For the first time in history, a situation is reached where our planet harbors more obese than underweight inhabitants. Obesity and related metabolic diseases have reached pandemic proportions over the last few decades and will probably place an ever-increasing burden on our global health systems. The evolution of our modern environment with ready accessibility to palatable, energy-dense foods in virtually unlimited quantity combined with a dominating sedentary lifestyle, has rapidly transformed Homo sapiens, in less than a century, into an obese species. Even worse, this transformation may already be impacting future generations via deleterious epigenetic programming.
Despite the fact that many people maintain a relatively constant energy balance over lifespan, the doubling of the prevalence of obesity and related metabolic diseases in just 30 years (faster than our genome may adapt) arguably implies that evolutionary-conserved homeostatic mechanisms tightly control the energy regulatory system biased toward efficiently defending energy stores over losses.
Consequently, understanding the complex processes, which promote excess weight gain and its homeostatic defense mechanisms, represents a major challenge to 21st century biomedical research.
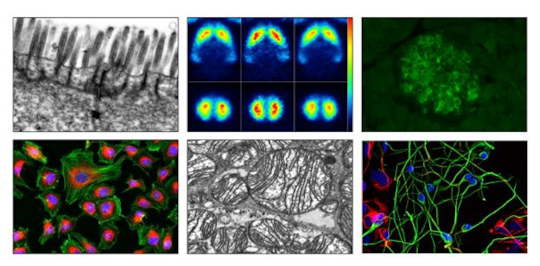
Top, left to right: Scanning Electron Microscope (SEM) image of intestinal epithelial cells, brain Positron Emission Tomography (PET) images, insulin staining of pancreatic islets. Bottom, left to right: fluorescence staining of subcellular compartments, mitochondria in brown adipose tissue, human stem cell derived progenitor cells and neurons (courtesy by the Institute of Reconstructive Neurobiology, UKB).
“All disease begins in the gut”
A main interest of our research is better understanding the impact of environmental factors and lifestyle (such as circadian rhythms, light-dark cycle, distress, diet, xenobiotics, bariatric surgery etc.) on human physiology and the sensory mechanisms by which the human body reacts to changes in the environmental conditions. Our particular interest is the interplay between the gut microbiome, the immune system, and their interaction with the central nervous system as perceptive entities of the human organism.
While the gut microbiota - the collection of microorganisms residing in the mammalian intestine - has long been considered as a predominantly passive member of the human ecosystem with mostly digestive functions, it is now clear that the microbiota is an integral part of systemic human physiology. The gut microbiota communicates with the central nervous system via the synthesis, processing and systemic release of metabolite signaling molecules to shape our endocrine and metabolic output system through mechanisms of nutrient handling and energy expenditure. Remarkably, many of our environmental influences could be shown to shape the microbial ecology and functional capacity, reasoning to hypothesize that therapeutic modulation of the microbiome might be harnessed to alter patient’s risk for the manifestation of a specific disease.
Our group is studying how environmental impacts regulate the inter-organ signaling network referred to as the ‘microbiota-gut-brain axis’, to define how targeted modulation of gut microbial composition and their metabolic activity affects neuronal circuits that govern energy homeostasis and endocrine function through output function on energy intake and its combustion within the adipose tissue.
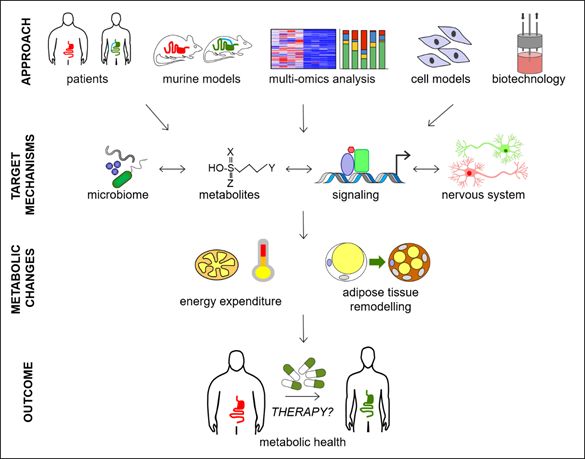
Figure 1: Our research implies the work with patients, corresponding animal models, cell culture paradigms, multi-omics approaches and aspects of innovative biotechnology to unravel and characterize novel molecular, cellular and inter-organ mechanisms that functionally converge to modulate human metabolism, and qualify for the development of novel therapeutic strategies.
Sitting at the intersection between basic and clinical research, we employ a multi-disciplinary approach combining cell culture, preclinical and clinical models to gain a holistic view on the molecular and cellular processes of the gut-brain axis that shape endocrine and metabolic health. Our lab is using an integrated physiology approach that relies greatly upon detailed in vivo phenotyping of genetically and non-genetically modified organisms. Together with Systems Biology approach, coupling integrated multi-omics analyses with cutting edge molecular cell biology tools, enables us to identify metabolic networks relevant for metabolic disease and translate findings into potential novel therapeutic targets for obesity and related endocrine diseases.
Research Projects
I. Basic Research
Bariatric surgery significantly alters the gut microbiota composition and function, which is linked to a considerably improved systemic metabolic profile and enhanced brown adipose tissue activity. Based on own experimental data, suggesting a causal role of the altered inter-organ crosstalk between gut microbiota and thermogenic adipose tissue in post-surgery weight loss and metabolic improvements, this DFG-funded project aims to determine the metabolic network correlating with thermogenic plasticity and identify active microbiota signaling candidates which may qualify to reactivate thermogenesis in human obesity.
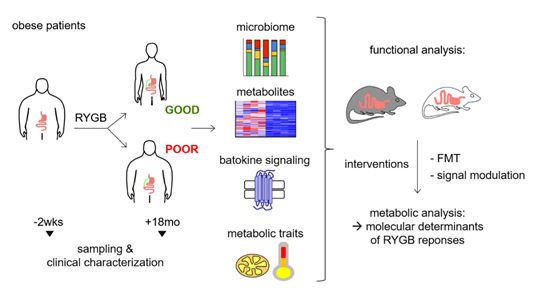
Figure 2: This diagram illustrates the translational approach investigating the role of RYGB-modulated gut-to-BAT signaling for treatment of human obesity.
This project is part of the novel Collaborative Research Center (CRC) TRR333 Brown and Beige Fat – Organ Crosstalk, Signaling and Energetics (BATenergy).
Please see consortium homepage: https://www.trr333.uni-bonn.de/ for further information.
The host gut provides a suitable habitat for particular bacterial groups, and in turn, gut microbiota contributes nutrient and energy supply to the host. Brown adipose tissue (BAT) and beige adipocytes facilitate mitochondrial thermogenesis to maintain host core body temperature during cold exposure and essentially adapt systemic metabolism to environmental needs. Recent evidence suggests that the composition of the gut microbiota alters during environmental temperature shifts to orchestrate energy homeostasis to the environmental condition. By depleting the gut microbiota through antibiotic cocktails, in this project we aim to identify molecular players and systemic signaling mechanisms that convey beneficial effects of targeted microbiota modulation on metabolic health. By combining in vitro and preclinical in vivo experiments with multi-omics analyses, we were able to identify the sulphur containing non-proteinogenic aminoacid TAURIN as main player that boosts metabolism of obese rodents, improves systemic metabolic parameters and reverts deleterious effects of high fat diet. Present projects utilizing animal models for obesity and feeding experiments aim to dissect the metabolic and genetic networks that regulate Taurine metabolism and its signaling network in the context of metabolic diseases.
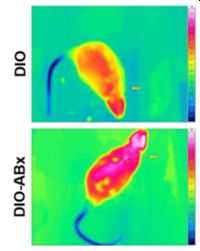
Figure 3: Quantification and representative infrared pictures of high-fat diet-induced obese (DIO) rats with (DIO-ABx) and without (DIO) ongoing antibiotics feeding (ABx), showing higher maximum interscapular brown adipose tissue (iBAT) temperature and surface temperature in microbiota depletion.
Type-2 diabetes (T2D) and obesity represent major risk factors for developing dementia-related disorders (such as Alzheimer’s disease). This project uses cell-culture based in vitro assays, high-content fluorescence microscopy and metabolomics to analyze the causative interactions between molecular profiles in patient serum and manifestation of neurodegenerative phenotypes. This approach will help us to identify differentially abundant metabolites or signaling molecules that affect dementia-related neuronal phenotypes (such as protein aggregation, oxidative stress, mitochondrial dysfunction, cell death etc) and thus represent promising targets for therapeutic interventions.
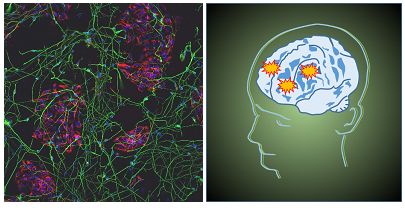
Figure 4: Type-2-diabetes represents a main risk factor for neurodegenerative diseases and dementia. Our project aims to explore the functional crosstalk between dysregulated glucose homeostasis and brain dysfunction. Left, human stem cell derived progenitor cells and neurons (courtesy by the Institute of Reconstructive Neurobiology, UK Bonn)

Figure 5. Metabolic characterization of patients implies state- of-the art analysis technologies, including (from left to right) infrared thermography, bioimpedance analysis and standardized body temperature modulation using cooling pads.
For the sake of translation of our experimental findings to human disease, a focus of our clinical research projects is to study the effect of weight loss strategies, including obesity surgery, diet regimens and/or next-generation anti-obesity drugs on the basal metabolic rates, energy homeostasis and thermogenesis in fat tissues. To this end, we utilize state-of-the-art patient-centered analytical methods such as indirect calorimetry, bioimpedance measurements and infrared thermography in combination with cooling pads. In addition, we closely interact with the BioBank Bonn (https://www.ukbonn.de/biobank/) in order to establish a comprehensive collection of tissues (liver, fat tissues), blood and stool samples from patients before and after interventions (e.g. surgery, drug application). These materials are used for comparative studies to identify key molecular factors modulating and enforcing therapy responses. We are convinced that these analyses will improve our understanding of molecular mechanisms underlying individual therapy outcome and open avenues for complementary future therapies.
Obesity therapy currently experiences the market entry of a plethora of novel anti-obesity drugs (AODs) that could revolutionize disease management strategies. While safety and efficacy of these tailored drugs (long-acting GLP-1 agonists, twincretins etc.) performed quite convincingly in clinical trials, their impact on cell type - specific disease pathways is largely uncharacterized. In particular, the impact of these drugs on immune cells and subsequent regulation of pro-/anti-inflammatory signaling remains unknown. Here we aim to describe the signaling processes initiated by AODs in patient- derived immune cell populations using peripheral blood mononuclear cells (PMBCs), stimulation with AODs or conditioned sera, and phospho-proteome analyses. Mapping of differentially phosphorylated proteins onto signaling networks will identify those functional modules that are affected by specific drug compounds. The results will help to describe drug effects in the context of obesity- associated inflammation.
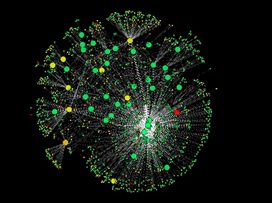
Figure 6. Protein interaction network representing >2,000 stress pathway-related proteins in human cells.
Adipocytes have a crucial role in energy homeostasis and metabolism due to their role as most important energy storage in mammals. While white adipocytes store excess energy as fat, so called brown adipocytes are specialized to consume energy and produce heat in a process termed non-shivering thermogenesis, which is essential to keep newborn mammals warm. Importantly, recent findings not only showed that also human adults possess metabolically active brown fat, but that its abundance and activity is positively correlated with cardiovascular health.
Metabolism of brown adipose tissue is research focus of the novel Collaborative Research Center (CRC) TRR333 Brown and Beige Fat – Organ Crosstalk, Signaling and Energetics (BATenergy).
Please see consortium homepage: https://www.trr333.uni-bonn.de/ for further information.

II. Clinical Research
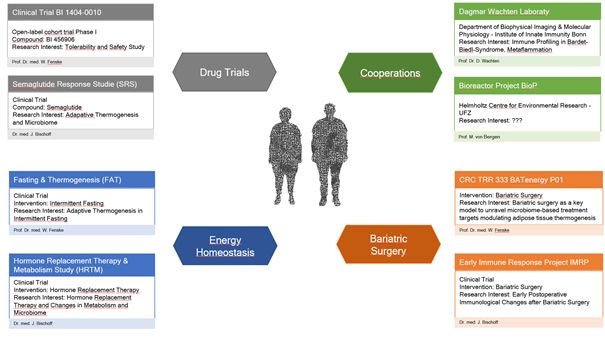
Figure 7. Overview of currently ongoing clinical research projects in our research center.
Based on recent outcome data from our experimental work, this DFG-funded clinical project (in cooperation with University of Graz, Austria) explores the functional impact of post-surgery microbiome alterations on host energy metabolism and glucose homeostasis via Fecal microbiota transfer (FMT) in human subjects. In a double-blinded proof-of-concept study, we here perform autologous versus allogenic FMT from lean and obese volunteers as well as from those after bariatric surgery into obese recipients. Additionally to effects on energy and glucose homeostasis, intervention-specific alterations on the microbiota composition and metabolite signature will be profiled by using multi-omics analyses. The major aim is to explore the scientific rational for targeted gut microbiota modulation in management of obesity and related metabolic diseases.
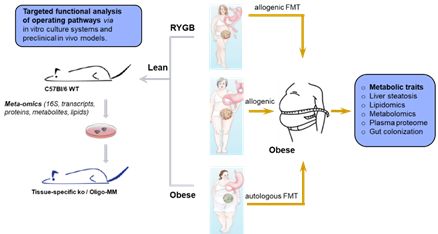
Figure 8. Overview of the clinical study design addressing autologous versus allogenic FMT to modulate gut microbiome in recipients.
The metabolic and energetic response to Glp-1-analogues is quite heterogeneous and sometimes limited by intolerable adverse effects. In this clinical study project, we aim to identify bacteria-derived modulators of systemic metabolite signaling that contribute to individual metabolic outcome and treatment tolerance of Glp-1 analogues in treatment-naïve type 2 diabetes and obesity. To this end, we will establish a biobank of patient stool samples before and after drug administration and analyze shifts in microbiome composition using 16S rRNA sequencing and metaproteomics in relation to metabolic outcome data.
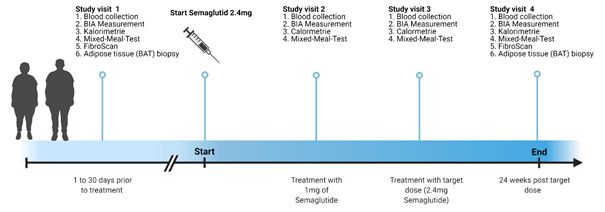
Figure 9. Summary of the estimated time line for the clinical study.
Intermittent Fasting (IF) or time-restricted eating (TRE) has shown positive effects on insulin resistance and oxidative stress in animal models of obesity. Here we aim to translate these findings into human subjects in order to unravel the effects of different fasting regimens on adaptive thermogenesis and brown adipose tissue activity in patients with obesity.
Transgender medicine represents an increasing field in modern health care systems, comprising the prevention, diagnosis and treatment of both physical and mental health issues for transgender subjects. While it is generally accepted that hormone replacement therapy and gender-affirming hormone therapy in transgender patients have profound effects on body composition and systemic cardio-metabolic control, mechanistic underpinnings remain unclear. The purpose of this study is to characterize changes in gut microbiota population architecture, body composition, energy combustion and systemic cardiometabolic consequences in response to therapy with sex hormones.
While bariatric surgery (i.e. the irreversible configuration of the architecture of the gastrointestinal tract) stands out amongst available weight management solutions in its efficacy and long-term character of therapeutic outcome in morbidly obese patients, implications of host immune responses in the orchestration of metabolic outcome after remain poorly addressed. This study seeks to better understand early onset (and weight loss independent) changes in blood immune cell populations after the surgical procedures using FACS-based analysis of peripheral blood derived immune cell populations and multiplex ELISA technology.
The drug BI 456906 (Bohringer-Ingelheim) represents a dual-acting agonist of glucagon-like peptide 1 (GLP-1) receptor and glucagon (GCG) receptor with potential wide ranging applications in the field of diabetes, overweight/obesity and liver diseases. This project encompasses a non-randomized, open-label, two part, parallel-cohort trial to evaluate 1) pharmacokinetics, safety and tolerability of a single dose of BI 456906 in patients with cirrhosis and varying degrees of hepatic impairment relative to healthy subjects and 2) safety and tolerability of multiple doses of BI 456906 in patients with overweight/obesity with cirrhosis and varying degrees of hepatic impairment relative to patients with overweight/obesity without hepatic impairment/cirrhosis.
According to preliminary evidence, obesity and metabolic syndrome share key pathological mechanisms with infertility disorders in both women and men. The rising prevalence of premature infertility features a major concern in western countries, affecting now about 10-20% of couples in Germany. While systemic insulin and neuronal leptin resistance are well-known mediators of compromised ovulation in women and attenuated spermatogenesis in men, cause-specific treatment modalities for improving reproductive success are not available.
Our research is focusing on environmental changes intertwined to the metabolic and immune system underlying perturbations on social reproduction in women and men. In clinical intervention studies, we also analyze the effect of novel drugs, targeting identified signaling pathways, on improved male and female fertility.
Cooperations
Institute of Innate Immunity, UK Bonn
Institute of Biochemistry and Molecular Cellbiology, UK Eppendorf
Computational Life Sciences, Life & Medical Sciences Institute (LIMES), University of Bonn
Helmholtz-Zentrum für Umweltforschung Leipzig
Rudolf Boehm Institute of Pharmacology and Toxicology, University of Leipzig
Graz Center for Microbiome Research, University of Graz, Austria
News
Our latest publication entitled ‘Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR-TGR5 crosstalk in diet-induced obesity’ (Münzker et al., Microbiome, 2022, in press) is featured as a ‘video byte’ by the Research Square Preprint Platform:
‚The gut microbiota is required to mediate the metabolic effects of gastric bypass in rats‘
Funding
Our research is supported by grants from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG), the Federal Ministry of Research and Education (Bundesministerium für Bildung und Forschung, BMBF), non-profit foundations and the Medical Faculty of the University of Bonn:
- Deutsche Forschungsgesellschaft/DFG (TRR333/1, AOBJ: 450149205, AOBJ: 624808, AOBJ: 667675, AOBJ: 676866)
- Bundesministerium für Bildung und Forschung/BMBF (FKZ: 01EO1501, IFB Adiposity Diseases)
- Die Else Kröner Fresenius Stiftung
Münzker J., Haase N., Till A., Sucher R., Haange S.B., (…), Pfeifer A., von Bergen M., Heeren J., Krügel U., and Fenske W.K. (2022) Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR-TGR5 crosstalk in diet-induced obesity
Microbiome (in press)
Fries C., Haange S.B., Rolle-Kampczyk U., Till A., Lammert M., Grasser L., Medawar E., Dietrich A., Hosretmann A., von Bergen, M., and Fenske W.K. (2022) Metabolic Profile and Metabolite Analyses in Extreme Weight Responders to Gastric Bypass Surgery
Metabolites (in press)
Bischoff J, Fries C, Heer A, Hoffmann F, Meyer C, Landsberg J, Fenske WK. (2022) It's Not Always SIAD: Immunotherapy-Triggered Endocrinopathies Enter the Field of Cancer-Related Hyponatremia. J Endocr Soc. 2022 Mar 9;6(5):bvac036.
Medawar, E., Haange, S.-B., Rolle-Kampczyk, U., Engelmann, B., Dietrich, A., Thieleking, R., Wiegank, C., Fries, C., Horstmann, A., Villringer, A., Bergen, M. von, Fenske, W.K. and Veronica Witte, A. (2021) Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Translational psychiatry 11 (1): 500. 10.1038/s41398-021-01620-3
Chen, J., Haase, N., Haange, S.-B., Sucher, R., Münzker, J., Jäger, E., Schischke, K., Seyfried, F., Bergen, M. von, Hankir, M. K., Krügel, U., and Fenske, W. K. (2021) Roux-en-Y gastric bypass contributes to weight loss-independent improvement in hypothalamic inflammation and leptin sensitivity through gut-microglia-neuron-crosstalk. Molecular Metabolism, 101214.
Muscate F, Woestemeier A., Gagliani N. (2021) Functional heterogeneity of CD4+ T cells in liver inflammation. Semin Immunopathol. 43(4):549-561.
Rheinwalt KP, Drebber U, Schierwagen R, Klein S, Neumann UP, Ulmer TF, Plamper A, Kroh A, Schipper S, Odenthal M, Uschner FE, Lingohr P., Trebicka J, Brol MJ. (2020) Baseline Presence of NAFLD Predicts Weight Loss after Gastric Bypass Surgery for Morbid Obesity. J Clin Med. 26;9(11):3430.
Fries, C. M., Bae, Y. J., Rayes, N., Sandner, B., Isermann, B., Stumvoll, M., Fagotto, V., Reincke, M., Bidlingmaier, M., Mandy, V., Kratzsch, J., and Fenske, W. K. (2020) Prospective evaluation of aldosterone LC-MS/MS-specific cutoffs for the saline infusion test. European Journal of Endocrinology 183, 191–201.
Palladino, V. S., Chiocchetti, A. G., Frank, L., Haslinger, D., McNeill, R., Radtke, F., Till, A., Haupt, S., Brüstle, O., Günther, K., Edenhofer, F., Hoffmann, P., Reif, A., and Kittel-Schneider, S. (2020) Energy Metabolism Disturbances in Cell Models of PARK2 CNV Carriers with ADHD. Journal of Clinical Medicine 9(12):4092.
Dohmen J, Praktiknjo M, Rudeloff A, Uschner FE, Klein S, Plamper A, Matthaei H, Rheinwalt KP, Wehner S, Kalff JC, Trebicka J, Lingohr P. (2020) Impact of sleeve gastrectomy and dietary change on metabolic and hepatic function in an obesity rat model - Experimental research. Int J Surg. 75:139-147.
Haange, S.-B., Jehmlich, N., Krügel, U., Hintschich, C., Wehrmann, D., Hankir, M., Seyfried, F., Froment, J., Hübschmann, T., Müller, S., Wissenbach, D. K., Kang, K., Buettner, C., Panagiotou, G., Noll, M., Rolle-Kampczyk, U., Fenske, W., and Bergen, M. von (2020) Gastric bypass surgery in a rat model alters the community structure and functional composition of the intestinal microbiota independently of weight loss. Microbiome 8(1):13.
Weykopf, B., Haupt, S., Jungverdorben, J., Flitsch, L. J., Hebisch, M., Liu, G.-H., Suzuki, K., Belmonte, J. C. I., Peitz, M., Blaess, S., Till, A., and Brüstle, O. (2019) Induced pluripotent stem cell-based modeling of mutant LRRK2-associated Parkinson's disease. The European Journal of Neuroscience 49, 561–589.
Brol MJ, Rösch F, Schierwagen R, Magdaleno F, Uschner FE, Manekeller S, Queck A, Schwarzkopf K, Odenthal M, Drebber U, Thiele M, Lingohr P., Plamper A, Kristiansen G, Lotersztajn S, Krag A, Klein S, Rheinwalt KP, Trebicka J. (2019) Combination of CCl4 with alcoholic and metabolic injuries mimics human liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 1;317(2):G182-G194
Christ-Crain, M., Bichet, D. G., Fenske, W. K., Goldman, M. B., Rittig, S., Verbalis, J. G., and Verkman, A. S. (2019) Diabetes insipidus. Nature Reviews Disease Primers 5, 54.
Plamper A, Goldmann G, Lingohr P., Horneff S, Dohmen J, Oldenburg J, Rheinwalt KP. (2019) First Case of Laparoscopic Mini-Gastric Bypass for the Treatment of Morbid Obesity in Severe Haemophilia A. Hamostaseologie. 39(2):208-210.
Sheng, C., Jungverdorben, J., Wiethoff, H., Lin, Q., Flitsch, L. J., Eckert, D., Hebisch, M., Fischer, J., Kesavan, J., Weykopf, B., Schneider, L., Holtkamp, D., Beck, H., Till, A., Wüllner, U., Ziller, M. J., Wagner, W., Peitz, M., and Brüstle, O. (2018) A stably self-renewing adult blood-derived induced neural stem cell exhibiting patternability and epigenetic rejuvenation. Nature Communications 9, 4047.
Fenske, W., Refardt, J., Chifu, I., Schnyder, I., Winzeler, B., Drummond, J., Ribeiro-Oliveira, A., Drescher, T., Bilz, S., Vogt, D. R., Malzahn, U., Kroiss, M., Christ, E., Henzen, C., Fischli, S., Tönjes, A., Mueller, B., Schopohl, J., Flitsch, J., Brabant, G., Fassnacht, M., and Christ-Crain, M. (2018) A Copeptin-Based Approach in the Diagnosis of Diabetes Insipidus. The New England Journal of Medicine 379, 428–439.
Fenske, W., Refardt, J., and Christ-Crain, M. (2018) Copeptin in the Diagnosis of Diabetes Insipidus. The New England Journal of Medicine 379, 1785–1786.
Jungverdorben, J., Till, A., and Brüstle, O. (2017) Induced pluripotent stem cell-based modeling of neurodegenerative diseases: a focus on autophagy. Journal of Molecular Medicine (Berlin, Germany) 95, 705–718.
Plamper A, Lingohr P, Nadal J, Rheinwalt KP. (2017) Comparison of mini-gastric bypass with sleeve gastrectomy in a mainly super-obese patient group: first results. Surg Endosc. 31(3):1156-1162.
Hankir, M. K., Kranz, M., Keipert, S., Weiner, J., Andreasen, S. G., Kern, M., Patt, M., Klöting, N., Heiker, J. T., Brust, P., Hesse, S., Jastroch, M., and Fenske, W. K. (2017) Dissociation Between Brown Adipose Tissue 18F-FDG Uptake and Thermogenesis in Uncoupling Protein 1-Deficient Mice. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 58, 1100–1103.
Hankir, M. K., Patt, M., Patt, J. T. W., Becker, G. A., Rullmann, M., Kranz, M., Deuther-Conrad, W., Schischke, K., Seyfried, F., Brust, P., Hesse, S., Sabri, O., Krügel, U., and Fenske, W. K. (2017) Suppressed Fat Appetite after Roux-en-Y Gastric Bypass Surgery Associates with Reduced Brain µ-opioid Receptor Availability in Diet-Induced Obese Male Rats. Frontiers in Neuroscience 10.
Hankir, M. K., Seyfried, F., Hintschich, C. A., Diep, T.-A., Kleberg, K., Kranz, M., Deuther-Conrad, W., Tellez, L. A., Rullmann, M., Patt, M., Teichert, J., Hesse, S., Sabri, O., Brust, P., Hansen, H. S., Araujo, I. E. de, Krügel, U., and Fenske, W. K. (2017) Gastric Bypass Surgery Recruits a Gut PPAR-a-Striatal D1R Pathway to Reduce Fat Appetite in Obese Rats. Cell Metabolism 25, 335–344.
Christ-Crain, M. and Fenske, W. (2016) Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nature reviews. Endocrinology 12, 168–176.
Hankir, M. K., Cowley, M. A., and Fenske, W. K. (2016) A BAT-Centric Approach to the Treatment of Diabetes: Turn on the Brain. Cell Metabolism 24, 31–40.
Hankir, M. K., Kranz, M., Gnad, T., Weiner, J., Wagner, S., Deuther-Conrad, W., Bronisch, F., Steinhoff, K., Luthardt, J., Klöting, N., Hesse, S., Seibyl, J. P., Sabri, O., Heiker, J. T., Blüher, M., Pfeifer, A., Brust, P., and Fenske, W. K. (2016) A novel thermoregulatory role for PDE10A in mouse and human adipocytes. EMBO Molecular Medicine 8, 796–812.
Lingohr P, Dohmen J, Matthaei H, Konieczny N, Hoffmann J, Bölke E, Wehner S, Kalff JC. (2016) Cytokine expression in the visceral adipose tissue after laparoscopic and conventional surgery in a rodent model. Eur J Med Res. 5;21:4
Börgeson, E., Johnson, A. M. F., Lee, Y. S., Till, A., Syed, G. H., Ali-Shah, S. T., Guiry, P. J., Dalli, J., Colas, R. A., Serhan, C. N., Sharma, K., and Godson, C. (2015) Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metabolism 22, 125–137.
Till, A., Saito, R., Merkurjev, D., Liu, J.-J., Syed, G. H., Kolnik, M., Siddiqui, A., Glas, M., Scheffler, B., Ideker, T., and Subramani, S. (2015) Evolutionary trends and functional anatomy of the human expanded autophagy network. Autophagy 11, 1652–1667.
Klinik
Prof. Dr. Wiebke Kristin Fenske
Head of Division of Endocrinology, Diabetes and Metabolism, Medical Clinic 1,
University Hospital Bonn
Principal Investigator BiOMe Research Center
Tel.: +49 (0) 228 / 287- 16789
E-Mail: Enable JavaScript to view protected content.
Labor
PD Dr. habil. Andreas Till
Lab Leader Division of Endocrinology, Diabetes and Metabolism, Medical Clinic 1
University Hospital Bonn Biomedical Center (BMZ)
Office: BMZ1, 3.OG, Room 025, Lab: BMZ, 2.OG, R218
Tel.: +49 (0) 228 / 287 -51751 (Office)
E-Mail: Enable JavaScript to view protected content.