AG Weisheit
PD Dr. med. Christina Katharina Weisheit
- +49228-287-11317
- Enable JavaScript to view protected content.
Focus of research
The overarching aim of my research is to elucidate the molecular and immunological mechanisms underlying aortic diseases and cardiac remodeling, with a particular emphasis on the interplay between aging, the immune response, and the microbiome. By developing innovative therapeutic drug delivery systems and establishing novel models for studying infective endocarditis, I aspire to identify new biomarkers and therapeutic strategies that enhance early detection, prevention, and treatment of aortic diseases, ultimately improving patient outcomes and survival rates.
Aortic diseases are often caused by chronic degenerative changes in aortic tissue, which jeopardize the integrity of all organ systems and significantly impact patient survival. Currently, the only widely accepted treatment for various forms of aortic disease, including aortic stenosis and aneurysms, is surgical intervention. Unfortunately, early detection, prevention, and causal treatments for these conditions remain lacking.
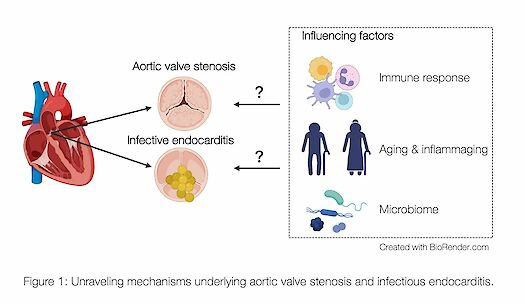
In recent years, research has begun to unravel the molecular and immunological mechanisms underlying these serious diseases, with the goal of identifying new therapeutic approaches and biomarkers. To facilitate this research, I utilize blood, stool, oral flora, and tissue samples from patients to investigate immunological reactions and the influence of the gut microbiome on aortic diseases (Fig. 1).
In addition to sterile aortic diseases, infective endocarditis (IE) represents another challenging aspect of our research interest. IE is defined as a bacterial infection of the heart, most commonly affecting the heart valves, with an increasing incidence in developed countries and a high mortality rate of up to 30%. Major complications include septic stroke and acute heart failure. Patients often present with only mild symptoms and lack direct IE-specific surrogates; echocardiography, CT, or PET-CT scans are frequently inconclusive, hindering early diagnosis and treatment. Risk factors for IE include cardiac procedures such as valve replacement or pre-existing innate or degenerative valvular disease. The most common bacteria causing IE in developed countries is Staphylococcus aureus (S. aureus), and its incidence is on the rise. The aortic valve is the most commonly affected valve in IE, particularly due to the prevalence of aortic valve stenosis in the elderly, which affects up to 2% of this population. In this context, our research includes the establishment of a novel, highly standardized model to investigate the underlying mechanisms of IE and the influence of aging and the microbiome.
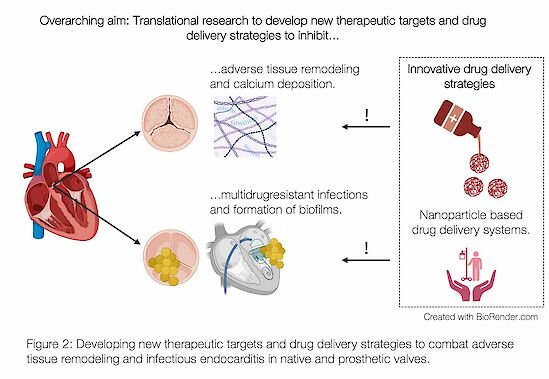
A significant area of my interest lies in understanding the impact of aging on the immune response and the progression of both sterile and infective aortic valve diseases. My group is focused on developing innovative therapeutic drug delivery systems for patients suffering from aortic diseases utilizing nanoparticle technology (Fig. 2).
Immunological Implications of Sepsis and Delir Development
In the joint CovidDataNet.NRW project, we contributed to optimizing risk stratification for patients who have survived sepsis and defining biomarkers through the analysis of epigenetic modifications in immune cells. We hypothesized that the increased mortality observed in patients following COVID-19 is due to impaired immune cell function, which we believe is rooted in epigenetic modifications. By comparing methylation profiles at different time points (differential methylation analysis), we aim to identify COVID-19 or sepsis-dependent changes in the methylation state of nucleated cells in the blood.
Delirium is common during critical illness and major surgical interventions and is associated with increased morbidity and mortality; however, its pathophysiology remains unclear. We hypothesize that dysfunctional cerebral autoregulation coincides with cerebral inflammation and contributes to the development of delirium.
- Investigation of the Immunological Mechanisms Underlying Aortic Valve Stenosis
This project focuses on understanding the immune responses associated with the development and progression of aortic valve stenosis. - Clarification of the Influence of the Microbiome on Aortic Diseases
This research aims to explore how the gut microbiome affects the pathophysiology of aortic diseases, potentially identifying new therapeutic targets. - Elucidation of the Immunological Mechanisms in Infective Endocarditis
This project seeks to uncover the immunological processes involved in infective endocarditis, contributing to a better understanding of the disease and its treatment. - Analysis of the Influence of Aging on Aortic Disease
This research examines how aging impacts immune responses and disease progression in patients with aortic diseases. - Development of Nanoparticles for Targeted Drug Delivery in Aortic Diseases
This project focuses on creating innovative nanoparticle-based systems designed for targeted drug delivery to improve treatment efficacy in aortic diseases. - Investigation of Epigenetic Modifications in Immune Cells and Their Role in Critical Illness
This project aims to explore the epigenetic modifications of immune cells that contribute to impaired immune function following COVID-19 and sepsis,
- Prof. Dr. Sebastian Zimmer (Herzzentrum, Universitätsklinikum Bonn)
- Prof. Dr. Verena Hörr (Herzzentrum, Universitätsklinikum Bonn)
- Prof. Dr. Bernardo Franklin (Institute of Innate Immunology, Universitätsklinikum Bonn)
- Prof. Dr. Achim Hörauf, PD Dr. Marijo Parcina (Institut für Mikrobiologie, Universitätsklinikum Bonn)
- Prof. Dr. Christian Kurts (Institut für Molekulare Medizin und Experimentelle Immunologie, Universitätsklinikum Bonn)
- Prof. Dr. Lars Fester, Prof. Dr. Stefanie Kürten (Anatomisches Institut, Universität Bonn)
- Prof. Dr. Annette Moter (Deutsches Biofilmzentrum, Charité Berlin)
- Prof. Dr. Holger Winkels, PD Dr. Felix Hoyer (Herzzentrum, Universitätsklinikum Köln)
- Prof. Dr. Markus Weigand (Klinik für Anästhesiologie und Intensivmedizin, Universitätsklinikum Heidelberg)
- Prof. Dr. Maria Grandoch (Institut für Translationale Pharmakologie, Universitätsklinikum Düsseldorf)
- PD Dr. Claudia Göttsch (Medizinische Klinik I, Kardiologie, RWTH Aachen)
- Prof. Elena Aikawa, MD, PhD (Center for Interdisciplinary Science, Harvard Medical School, Boston USA)
Leitung / Ansprechpartner inkl. Kontaktdaten
- PD Dr. med. Christina Katharina Weisheit
- Member of the Excellence Cluster Immunosensation2
- Member of the ACCENT Program Bonn for Advanced Clinician Scientists
- 0228-287-11317
- Enable JavaScript to view protected content.
Mitarbeiter und Mitarbeiterinnen
- Jan Lukas Kleiner
- Dr. med. Benedikt Bartsch
- Mariam Louis Nazir
- Mina Girgis
Masterstudierende
- Fatemeh Hajikazemi
Doktoranden und Doktorandinnen
- Cand med. Lennart Buchen
- Cand med. Moritz Altrogge
- Cand med. Lisa Slater

DFG Einzelförderung: Projektnummer: 535107899
SFB TRR259 Projekt A01 Fördernummer: 397484323 – Projektnummer: 426093965
DLR Einzelförderung
CovidDataNet.NRW Standort Bonn
- Ziehe D, Wolf A, Rahmel T, Nowak H, Haberl H, Bergmann L, Rump K, Dyck B, Palmowski L, Marko B, Witowski A, Willemsen KM, Pfaender S, Eisenacher M, Anft M, Babel N, Bracht T, Sitek B, Bayer M, Zarbock A, von Groote T, Putensen C, Ehrentraut SF, Weisheit C, Adamzik M, Unterberg M, Koos B. Exploring the relationship between HCMV serostatus and outcomes in COVID-19 sepsis. Front Immunol. 2024 May 8;15:1386586. doi: 10.3389/fimmu.2024.1386586.
- Niepmann ST, Willemsen N, Boucher AS, Stei M, Goody P, Zietzer A, Bulic M, Billig H, Odainic A, Weisheit CK, Quast C, Adam M, Schmidt SV, Bakhtiary F, Jansen F, Nickenig G, Latz E, Zimmer S. Toll-like receptor-3 contributes to the development of aortic valve stenosis. Basic Res Cardiol. 2023 Feb 1;118(1):6. doi: 10.1007/s00395-023-00980-9.
- Rajlic S, Surmann L, Zimmermann P, Weisheit CK, Bindila L, Treede H, Velten M, Daiber A, Duerr GD. Fatty Acid Amide Hydrolase Deficiency Is Associated with Deleterious Cardiac Effects after Myocardial Ischemia and Reperfusion in Mice. Int J Mol Sci. 2022 Oct 21;23(20):12690. doi: 10.3390/ijms232012690.
- Kleiner JL, Köpke O, Faron A, Zhang Y, Cornelssen J, Coburn M, Frede S, Eichhorn L, Weisheit CK. Characterization of transverse aortic constriction in mice based on the specific recruitment of leukocytes to the hypertrophic myocardium and the aorta ascendens. Mediators Inflamm. 2021 Nov 3;2021:1376859. doi: 10.1155/2021/1376859. eCollection 2021.
- Muenster S, Zschernack V , Dierig B, Frede S, Baumgarten G, Coburn M, Putensen C, Weisheit CK. Vancomycin and daptomycin modulate the innate immune response in a murine model of LPS-induced sepsis. Int J Immunopathol Pharmacol. Jan-Dec 2021;35:20587384211031373. doi: 10.1177/20587384211031373.
- Weisheit CK, Kleiner JL, Rodrigo MB, Niepmann ST, Zimmer S, Dürr GD, Coburn M, Kurts C, Frede S, Eichhorn L. CX3CR1 is a prerequisite for the development of cardiac hypertrophy and left ventricular dysfunction in mice upon transverse aortic constriction. PloS One. 2021 Jan 07; 16(1): e0243788 https://doi.org/10.1371/journal.pone.0243788.
- Jobin K, Stumpf NE, Schwab S, Eichler M, Neubert P, Rauh M, Adamowski M, Babyak O, Hinze D, Sivalingam S, Weisheit CK, Hochheiser K, Schmidt S, Meissner M, Garbi N, Abdullah Z, Wenzel U, Hölzel M, Jantsch J, Kurts C. A high-salt diet compromises the neutrophil response against bacterial infections through hormonal perturbations. Science Translat Med. 2020 Mar 25;12(536):eaay3850. doi: 10.1126/scitranslmed.aay3850.
- Weisheit CK, Klüners A, Wild L, Casalter A, Heilmann-Heimbach S, Sivalingam S, Kleiner JL, Ehrentraut S, Hoeft A, Frede S, Ehrentraut H. Sustained immunoparalysis in endotoxin-tolerized monocytic cells. Mediators Inflamm. 2020 Jun 13;2020:8294342. doi: 10.1155/2020/8294342.
- Graefe C, Eichhorn L, Wurst P, Kleiner J, Heine A, Panetas I, Abdulla Z, Hoeft A, Frede S, Kurts C, Endl E, Weisheit CK. Optimized Ki-67 staining in murine cells: a tool to determine cell proliferation. Mol Biol Rep. 2019 Aug;46(4):4631-4643. doi: 10.1007/s11033-019-04851-2.
- Ehrentraut H*, Weisheit C*, Scheck M, Frede S, Hilbert T. Experimental murine acute lung injury induces increase of pulmonary TIE2-expressing macrophages. J Inflamm (Lond). 2018 Jun 14;15:12. doi: 10.1186/s12950-018-0188-5. eCollection 2018. *these authors contributed equally to this work.
- Weisheit C, Zhang Y, Faron A, Köpke O, Weisheit G, Steinsträsser A, Frede S, Meyer R, Boehm O, Hoeft A, Kurts C, Baumgarten G. Ly6C(low) and not Ly6C(high) macrophages accumulate first in the heart in a model of murine pressure-overload. PLoS One. 2014 Nov 21;9(11):e112710. doi: 10.1371/journal.pone.0112710. eCollection 2014.
- Schiwon M*, Weisheit C*, Franken L, Gutweiler S, Dixit A, Meyer-Schwesinger C, Pohl JM, Maurice NJ, Thiebes S, Lorenz K, Quast T, Fuhrmann M, Baumgarten G, Lohse MJ, Opdenakker G, Bernhagen J, Bucala R, Panzer U, Kolanus W, Gröne HJ, Garbi N, Kastenmüller W, Knolle PA, Kurts C*, Engel DR*. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium *these authors contributed equally to this work. Cell. 2014 Jan 30;156(3):456-68. doi: 10.1016/j.cell.2014.01.006.
- Billig S, Hein M, Uhlig M, Schumacher D, Thudium M, Coburn M, Weisheit CK. Anesthesia for aortic valve stenosis: Anesthesiological management of patients with aortic valve stenosis during noncardiac surgery | [Anästhesie bei Aortenklappenstenose: Anästhesiologisches Management von Patienten mit Aortenklappenstenose bei nichtkardiochirurgischen Eingriffen]
- Weisheit CK, Geerling G, Holz FG, Coburn M. High-Impact Actions to Reduce the Carbon Dioxide Footprint in an Ophthalmic Operation Room: A Narrative Review. Ophthalmologica 2023. 10.1159/000533444
- Quast C, Weisheit CK. Basic Science und Klinik - wie geht das überhaupt? Trillium Immunologie 2020; 4(1):47–51.
- Weisheit CK, Engel DR, Kurts C. Dendritic Cells and Macrophages: Sentinels in the Kidney. Clin J Am Soc Nephrol. 2015 Jan 7. pii: CJN.07100714. doi: 10.2215/CJN.07100714


















