Wilhelm Group
Scientific co-director / group leader

Tel.: +49 228 287-51721
Fax: +49 228 287-16094
North Zone, Building No. 12
1st Floor, Room 1G402
We are studying the metabolic control of mucosal immune cells to better understand what distinguishes their protective function in infection from immune-mediated pathology in chronic inflammation. Specifically, we aim to untangle how dietary restriction and fasting affects the immune system and how exogenous and endogenous metabolites influence barrier immune cells. We have found that a ketogenic diet can be used as an effective dietary intervention strategy for the treatment of chronic airway inflammation and that ketogenesis triggered by severe respiratory infections supports T cell function by providing ketone bodies as an alternative carbon source. Our central goal is to understand the metabolic, nutritional and physiological consequences of westernization on protective immunity and immune-mediated pathologies.
Over the last decades the epidemiology of immune diseases has changed considerably in the Western World. Thus, the gradual eradication of many infectious diseases coincides with an increase of chronic inflammatory conditions such as asthma or inflammatory bowel disease (IBD). The core of our scientific interest is to understand the underlying mechanisms and causes for the steadily increasing incidents of chronic inflammation. Recent advances identified a group of innate immune cells, termed innate lymphoid cells (ILC), which reside at different barrier surfaces, like the lung or intestine. The major task of ILC is the protection and maintenance of the tissue barrier by mediating anti-bacterial responses, tissue repair or anti-helminth immunity. However, besides their protective function, chronic activation of ILC promotes inflammation and ILC have been implicated in inflammatory disorders such as allergies and asthma and IBD. In general, the etiology of these barrier diseases is poorly understood but changes in life-style coinciding with westernization, such as abundant nutritional uptake and increased hygiene, leading to an overall absence of parasitic infections are suggested to play a role in disease development. In contrast, people in the developing world still experience conditions of malnutrition and increased exposure to infections but an overall absence of immune-mediated pathologies and chronic inflammatory diseases such as asthma or IBD. Our key hypothesis is that chronic pathogenic immune responses are shaped by western life style and are metabolically differentially regulated from protective immune responses in helminth infections. By studying both, protective immune responses to infections but also chronic inflammation, we will be able to delineate the metabolic mechanisms controlling protective and pathogenic immune responses.
The Metabolic Control of Protective or Pathogenic ILC Responses
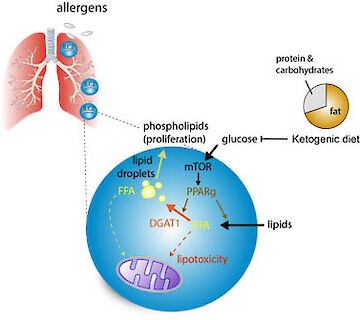
The Metabolic Control of Protective or Pathogenic ILC ResponsesInvestigating the metabolic pathways in control of protective ILC2 functions in mice we discovered that anti-helminth immunity critically depends on fatty acid (FA) metabolism, while glucose is dispensable (Wilhelm et al. 2016). These findings suggested, that the protective ILC2 response tailored towards repair in the context of infections is driven by catabolic FA metabolism (fatty acid oxidation). The important implication of this discovery is that ILC2-mediated tissue repair and anti-helminth immunity are maintained in settings of low glucose availability and malnutrition. Both, malnutrition and helminth infections are largely absent in the western world, with unknown consequences for ILC-mediated immune responses. Our more recent work now shows that in airway inflammation pathogenic ILC2 require increased uptake of both glucose and lipids, which drive extensive proliferation and pathogenicity. In contrast to the metabolic profile of protective ILC2 in helminth infections, we found that externally acquired fatty acids are used for anabolic processes to build up cellular membranes. Thus, we speculated that pathogenic activation might rely on the availability of excess nutrients. We discovered ketogenic diets as a potent dietary intervention strategy to treat ILC-driven pathologies such as allergic asthma (Karagiannis et al. 2020). Since ketogenic diets mimic a state of fasting and nutrient deprivation this may explain how periods of malnutrition have counterbalanced the development of chronic inflammation in the past and how increased incidence of immune pathology in the Western World may be driven by increased consumption of carbohydrate and fat. As a follow up to our published studies, we are aiming to better understand the importance of lipid metabolism in ILC biology and the positive effect of dietary restriction, fasting metabolism and ketogenesis and pathogenic activation of immune cells.
Project related publications:
Karagiannis F, Kharabi Masouleh S, Wunderling K, Surendar J, Schmitt V, Kazakov A, Michla M, Hölzel M, Thiele C & Wilhelm C. Lipid droplet formation drives pathogenic type 2 innate lymphoid cells in airway inflammation. Immunity 2020; 52(4):620-634.
Wilhelm C, Harrison OJ, Pelletier M, Spencer SP, Urban JF Jr, Ploch M, Ramalingam TR, Siegel R, Belkaid Y. Critical role of fatty acid metabolism in ILC2 mediated barrier protection during malnutrition and helminth infection. J Exp Med 2016: 213(8):1409-18.
Infection-induced ketogenesis and the efficacy of ketone bodies and ketogenic diets in severe respiratory viral infections.
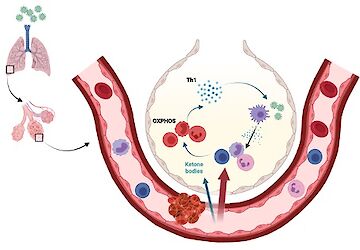
Here we aim to better understand the beneficial effects of nutrient restriction in infections. While both animals and humans infected with influenza develop robust ketogenesis, this response is absent in patients infected with Sars-CoV2 (Karagiannis et al. Nature 2022). Our published work shows that the liver-derived ketone body beta-hydroxypyruvate produced in the context of infection-related anorexia enhances the antiviral T cell immunity. Following our published study, we are further investigating the beneficial effect of ketogenesis and the production of BHB but also how infection-induced ketogenesis is regulated. In this project we are following the idea that ketone bodies produced from lipids in the context of infection-induced anorexia can directly prevent aberrant activation of immune cells while promoting protective immunity.
COVID-19
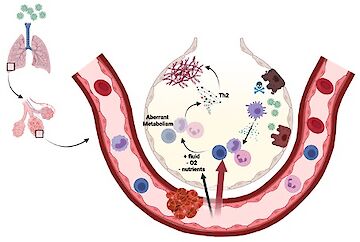
Ketone bodies in COVID-19
Project related publications:
Karagiannis F, Peukert K, Surace L, Michla M, Nikolka F, Fox M, Weiss P, Feuerborn C, Maier P, Schulz S, Al B, Seeliger B, Welte T, David S, Grondman I, de Nooijer AH, Pickkers P, Kleiner JL, Berger MM, Brenner T, Putensen C; Bonn COVIMMUNE Consortium, Kato H, Garbi N, Netea MG, Hiller K, Placek K, Bode C, Wilhelm C. Impaired ketogenesis ties metabolism to T cell dysfunction in COVID-19. Nature 2022; 609(7928):801-807
Wilhelm C, Surendar J, Karagiannis F. Enemy or ally? Fasting as an essential regulator of Immune resposnes. Trends in Immunology 2021; 42(5):389-400.
Harnessing Metabolites to Modulate Infection and Chronic Inflammation
Correlation studies and experimental evidence suggest that increased hygiene and westernization promote chronic inflammatory conditions. Respiratory viral infections induce a transient state of fasting and ketogenesis. Furthermore, helminth infections impose changes on host metabolism, which may reflect the need of parasites to gain sufficient access to nutrients, putting intestinal helminths in direct competition with the host and microbiota. Thus, to understand the impact of increased hygiene we follow the hypothesis that infections prevent pathogenic activation of immune cells and the development of chronic inflammation by imposing a metabolic restrain on the host. In this regard we study the immunomodulatory capacity of host-, diet- or microbiota-derived metabolites we find altered in the context of infection and test their potential to prevent immune-mediated pathologies in mice and humans.
Group members
Dr. David Fernando Colón Morelo, PostDoc
Phone: +49 228 287-51730
Maria Dovermann, Doctoral candidate
Phone: +49 228 287-51730
Dr. Fotis Karagiannis, PostDoc/Research Associate
Phone: +49 228 287-51730
Dr. Timothy McCulloch, PostDoc
Phone: +49 228 287-51730
Roman Rombo, Technician
Phone: +49 228 287-51720
Laetitia Sanelli, Doctoral candidate
Phone: +49 228 287-51720
Carola Sarici, Technician
Phone: +49 228 287-51730
Dhruvi P. Shah, Doctoral candidate
Phone: +49 228 287-51720
Jonathan Scheler, Student assistant
Phone: +49 228 287-51720
Maria Rafailia Theodorou, Doctoral candidate
Phone: +49 228 287-51720
Patricia Weiss, Technician
Phone: +49 228 287-51730
Chantal Wientjens, Doktorandin
Phone: +49 228 287-51720
Jobs available at the Wilhelm Group
We are always interested in recruiting highly motivated students and postdocs and thus encourage applications from prospective candidates at anytime.