Lutetium-177-PSMA therapy
Therapy for patients with metastasized prostate cancer with Lutetium-177-PSMA has been established in our hospital already in 2014. Lu-177-PSMA is a new therapy option for patients with metastasized prostate cancer when antihormonal treatments and chemotherapy are no longer effective. In contrast to other radionuclide therapy forms for prostate cancer, such as radium-223-dichloride (Xofigo®) or samarium-153, which only target bone metastases of prostate cancer, Lu-177-PSMA can treat all tumour localizations such as e.g. bone metastases, lymph node metastases and other organ metastases.
PSMA is an abbreviation for Prostate Specific Membrane Antigen. PSMA is a protein that can regularly be found on the surface of prostate cancer cells. For use in nuclear medicine diagnostics and therapy, radioactively labelled substances that specifically bind to PSMA (key-and-lock principle) have been developed. Gallium-68-labelled PSMA is usually employed for diagnostic purposes in Ga-68-PET/CT imaging, whereas Lutetium-177-labelled PSMA is the substance of choice for therapeutic applications. Due to the specific binding of these compounds to PSMA on the surface of the tumour cells, the radioactive nuclide Lu-177 accumulates in the malignant tumour and the emitted beta-radiation kills the tumour cells with high efficacy, just like an internal radiotherapy. Due to the very short range of the beta-radiation of Lu-177 in human tissue (only about 1 mm), very high radiation doses can be delivered directly to the tumour in a highly conformal manner, meaning that the surrounding healthy tissue is mostly spared from the effects of the radiation.
From well over 1000 cycles of therapy performed in our institution, we know that up to 70% of patients (who do not have any other therapy options available anymore) benefit from Lu-177-PSMA therapy, which results in shrinkage of tumour masses, decrease of the tumour marker PSA and mitigation of pain and other symptoms.
Patients who are initially diagnosed with prostate cancer often undergo surgical treatment or radiotherapy. Despite optimal initial treatment relapses can occur, which in many cases can be controlled with e.g. antihormonal treatment for an extended time period. However, the tumour cells can develop resistance against these antihormonal treatments and patients might have to undergo chemotherapy, to which the tumour cells also can develop resistance during the course of treatment.
This scenario is a typical indication for Lu-177-PSMA therapy. It is a new and promising therapy option for patients with prostate cancer when no other therapy options are available. Lu-177-PSMA therapy is not a first-line therapy. This is also recommended by the S3-guidelines for prostate cancer (recommendation 6.45).
Lu-177-PSMA therapy is only possible when the tumour and metastases are PSMA-positive.
This is assessed by a Ga-68-PSMA PET/CT examination before Lu-177-PSMA therapy (figure 1). Of course, the Ga-68-PSMA PET/CT examination can also be performed in our institution.
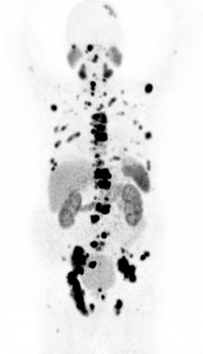
Figure 1: Ga-68-PSMA PET/CT showing several PSMA-positive metastases of prostate cancer.
Lu-177-PSMA therapy is performed on our inpatient radionuclide therapy ward (“Station Winkler”) in the University Hospital Bonn. After an initial physical examination, laboratory blood values are assessed. Furthermore, the kidney function is assessed by a scintigraphic examination (MAG3 renal scan) to exclude a urinary obstruction. About 20 – 30 minutes before application of Lu-177-PSMA, cooling of the salivary glands with ice packs is started (see figure 2), which reduces blood flow to the salivary glands to minimize unwanted uptake of Lu-177 in the salivary glands. Cooling of the salivary glands is continued for about 4 hours after the application of Lu-177-PSMA.
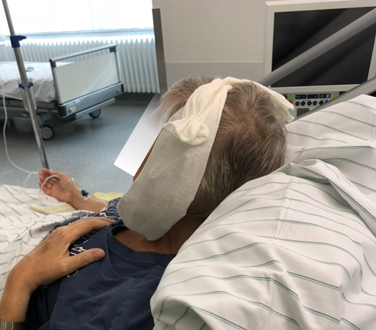
Figure 2: Cooling of the salivary glands.
Lu-177-PSMA is injected intravenously, followed by an infusion of about 500 – 1000 mL of fluid. Due to radiation safety regulations, patients have to stay on the inpatient therapy ward for 48 hours after injection of Lu-177-PSMA. After the therapy, distribution of the therapeutic agent is assessed by a whole body scintigraphy to document tumour uptake (see figure 3). All personal belongings that you may have brought with you for your stay on the inpatient ward (e.g. books, laptop, smartphone, tablet, clothing, etc.) can be taken home directly after therapy. All patient rooms are equipped with personal flat-screen TVs. Wifi is available.
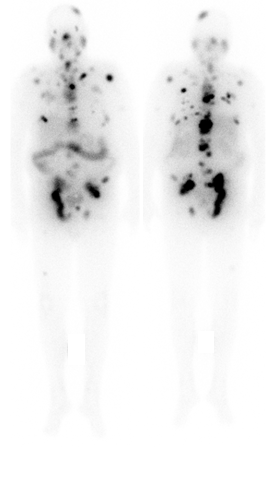
Figure 3: Whole body scintigraphy 24 hours after application of Lu-177-PSMA therapy, showing excellent uptake of the therapeutic agent in the metastases.
Lu-177-PSMA therapy consists of several cycles, initially often between 3 – 5 cycles, depending on tumour burden and individual response to therapy. The therapy cycles take place every 6 – 8 weeks.
Lu-177-PSMA therapy can be repeated if there is a disease relapse after initial response to Lu-177-PSMA therapy.
Usually, Lu-177-PSMA therapy is tolerated very well and it does not lead to serious acute side effects. However, blood values (kidney function and blood cell counts) have to be checked before therapy and periodically after therapy. According to several retrospective studies, significant impairment of kidney function occurs only very rarely, even after several cycles of Lu-177-PSMA therapy. Significant changes of blood cell counts may occur in up to 10% of patients after several cycles of Lu-177-PSMA therapy.
You or your treating physician can contact our therapy secretary’s office (contact data see below) to organize Lu-177-PSMA therapy. After an initial check of your disease history and the current disease state (e.g. current imaging studies), a Ga-68-PSMA PET/CT examination can be organized in our institution (not necessary if a PSMA PET/CT examination has already been performed recently). An interdisciplinary tumour board focused on urological tumours in our institution will then check if Lu-177-PSMA therapy is the recommended therapy for you (this is not necessary if a recommendation of an interdisciplinary tumour board of another institution is already available).
Therapy secretary office / enquiries
Mrs. Oregan Vautey
Tel: +49 (0)228 287 16171
Email:
Therapy Ward
Tel: +49 (0)228 287 16855
Fax: +49 (0)228 287 19107



















