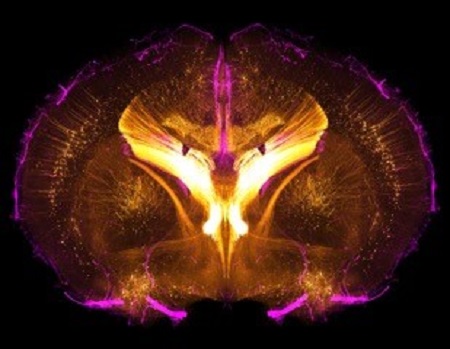
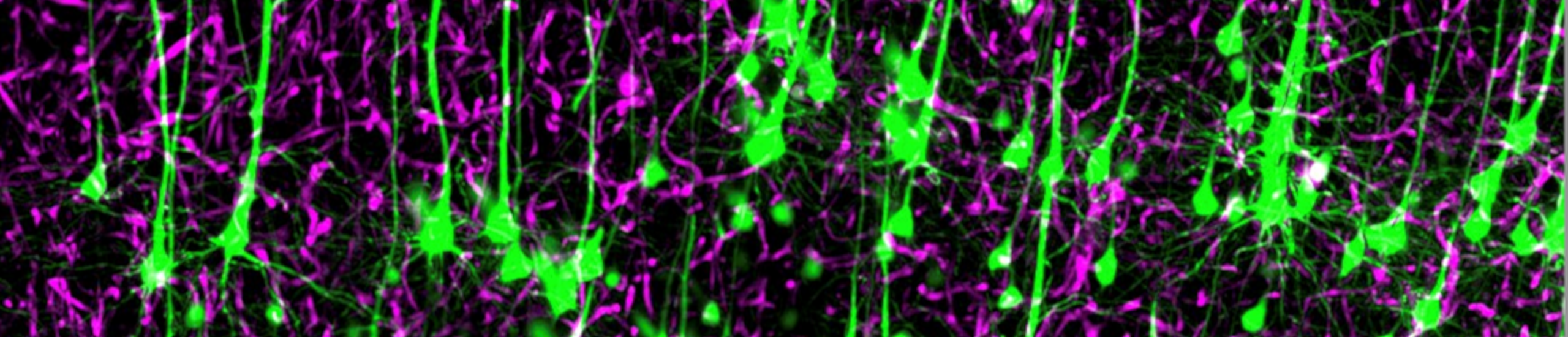
Neurovascular Interaction Group
Research in our group is focussed on neurovascular interactions in development, homeostasis and disease.
We aim to understand how the vasculature of the central nervous system forms and acquires its specific organotypic features. We aim to characterize the molecular signals that neural cells and blood vessels use to communicate with each other in order to build and maintain highly precise functional networks. We are also studying the vascular changes that occur in CNS pathologies and how vascular dysfunction and impaired cross-talk with the neural parenchyma can contribute to the neurological phenotypes.
For this, we are using a combination of mouse genetics, viral gene targeting, organotypic cultures, cell biology, biochemistry and molecular biology approaches.
Determining how the vascular system influences neuronal wiring, function and dysfunction will shed new light on the understanding of the molecular control of neural development and maintenance, and provide new paths for deepening our understanding of neurological diseases.
Specific projects
The oligo-vascular interface: understanding its properties and functions (OLI.VAS) ERC-CoG (European research council consolidator grant).
Recent research indicates that apart from delivering oxygen and nutrients, blood vessels are active regulators of organ development and function. Our recent work supports the existence of an oligo-vascular interface where vessels directly control the specification of oligodendrocyte precursor cells (OPCs) from neural progenitors (Paredes et al, Nat Neurosci, 2021). Once specified OPCs and blood vessel interactions are also important for further OPC differentiation and function. Still, the molecular mechanisms of bi-directional signaling that govern those oligo-vascular interactions remain elusive. The OLI.VAS project is now tackle the challenge to characterize in depth the interaction between OLs and the vasculature during development and demyelinating diseases.
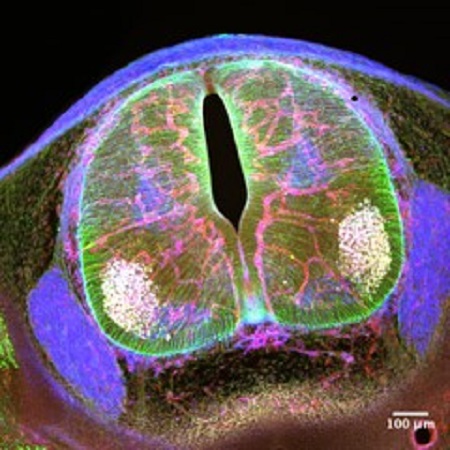
Brain endothelial cell-derived angiocrine factors and their role in neurodevelopment. Funded project within the CRC1366 (project A02).
Formation and function of the central nervous system requires the integration and coordination of multiple cell types including neurons, glia, microglia and vascular cells. Although initially avascular, the CNS becomes vascularized at the same time as neural cells are also developing. How the growing vasculature influences neurodevelopmental processes is not well understood. In this project we aim to tackle this question by characterizing the active response that endothelial cells exert on developing neural cells during brain formation. Results from this project will bring a better understanding of the fundamental principles that govern CNS formation and function, crucial for advancing our understanding of neurodevelopmental disorders.
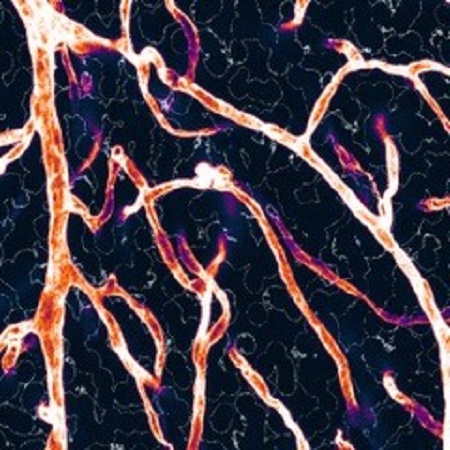
Targeting Autophagic Networks and the lysosome in Cerebral Small Vessel Disease Tackle-CSVD ((ERA-NET-Neuron)
This project is part of an international consortium network formed by four groups: Prof. Petzold (DZNE, Germany); Prof. Yaniv (Weismann Institute of Science, Israel); Dr. Plaza-Zabala (Univ. Vasque Country, Spain) and Prof. Ruiz de Almodovar (Uni. Bonn, Germany).
Cerebral small-vessel disease (CSVD) refers to a spectrum of sporadic or hereditary chronic pathological alterations of cerebral arterioles, capillaries and venules mostly within the white matter, which lead to vessel rarefication, demyelination, blood-brain barrier (BBB) dysfunction, glial activation and axonal loss. CSVD is responsible for ~30% of ischemic strokes and is a common cause of functional disability and cognitive impairment, imposing an enormous socio-economic burden. Thus, there is the urgent need to develop new therapeutic approaches to treat CSVD. While the majority of studies have focused on characterizing the vascular changes occurring in CSVD, the contribution of cells of the neural parenchyma to the disease remain incompletely understood. In this proposal we hypothesize that lysosomal-autophagy impairment centrally contributes to demyelination, glial activation and neuronal loss in CSVD. To work on this hypothesis we combine expertise and knowledge of 4 research groups, ranging from the fields of neurology, vascular biology, neurovascular biology and lysosomal biology. Research in this proposal combines CSVD patient samples and mouse models, zebrafish models and in vitro and biochemical approaches.
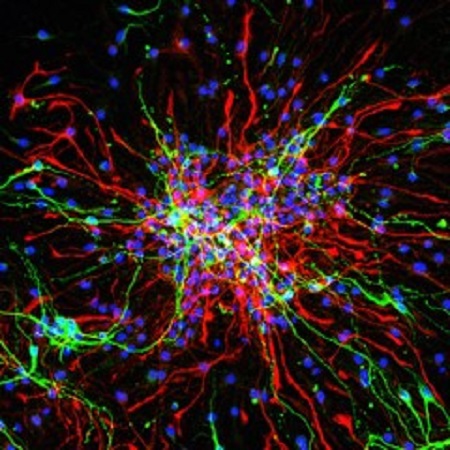
Pain-associated epigenetic alterations and spinal sensitization: role of the neurovascular unit. Funded project within the SFB1158 (project A08).
This is a shared project with Prof. Mauceri (Univ. Heidelberg, Germany), Dr. Tappe-Teodor (Univ. Heidelberg, Germany) and Prof. Ruiz de Almodovar (Uni. Bonn, Germany).
Chronic pain is defined is pain that last for more than 3 months. Central sensitization – alterations in central nervous system processing – is associated to different conditions of chronic pain. Here in this project we aim to explore the potential alterations occurring in the CNS the neurovascular unit in the context of chronic pain. How the NVU might contribute to pain sensitivity is also not known. Here we are investigating the neurovascular alterations occurring in the spinal cord upon different pain stimuli and how they contribute to pain chronicity.