ATLAS/NOA-29 trial
Schneider et al., 2025, BMC Cancer
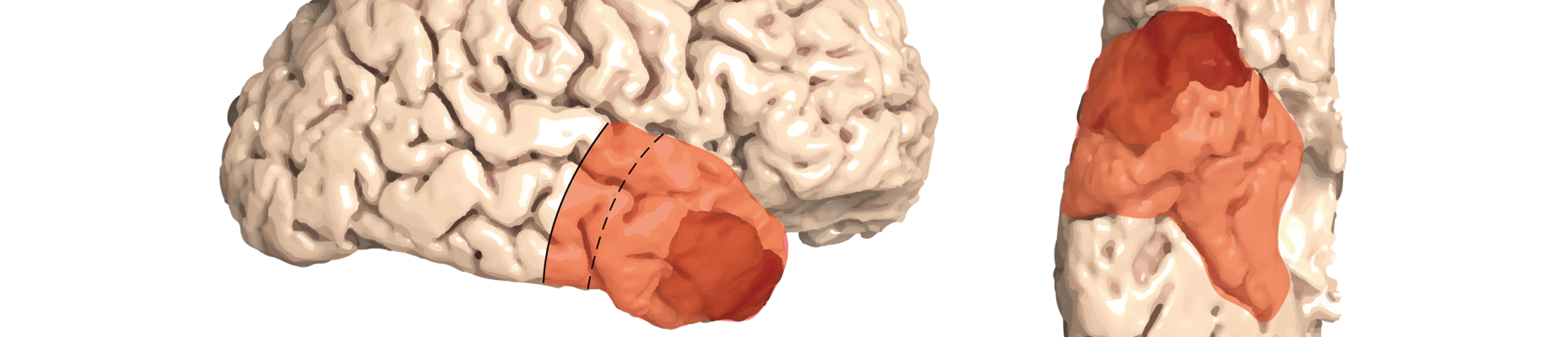
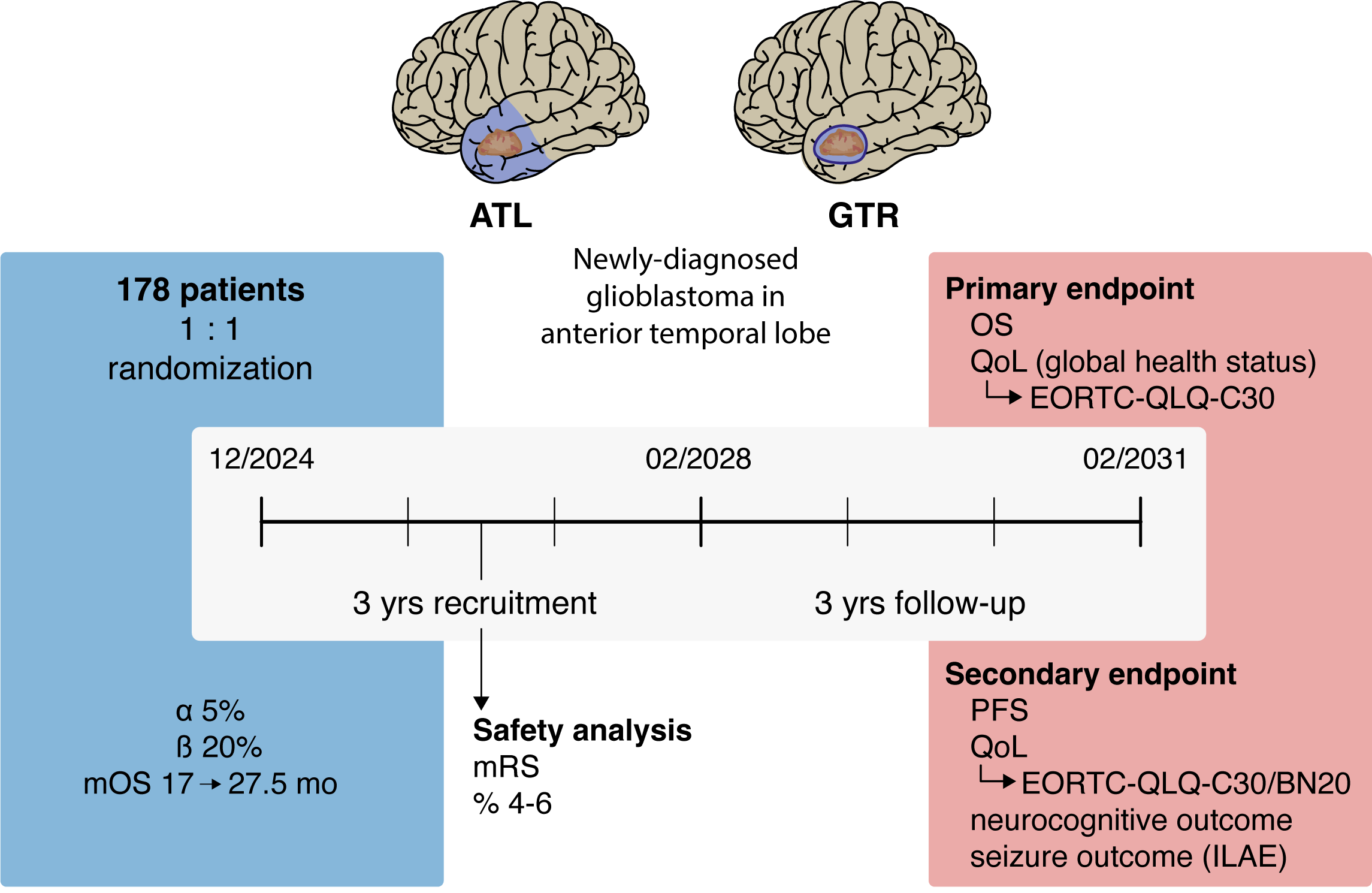
Anterior Temporal Lobectomy (ATL) versus gross-total resection (GTR) in temporal glioblastoma surgery
Objectives and endpoints
The primary objective is to prove superiority of anterior temporal lobectomy (ATL) compared to gross total resection (GTR) in patients with newly-diagnosed glioblastoma of the temporal lobe. Superiority will be defined as significant differences in the primary endpoint overall survival (OS) and non-inferiority regarding the co-primary endpoint quality of life (QoL; domaine „global health“ of the EORTC QLQ C30 questionnaire). Secondary endpoints include progression-free survival (PFS), functional outcome (as measured by modified Rankin Scale (mRS) and Karnofsky Performance Score (KPS)), seizure outcome, neurocognitive outcome (short test battery focused on temporal lobe functions) and evaluation of all QoL domains of EORTC QLQ C30 and BN20 questionnaires over time.
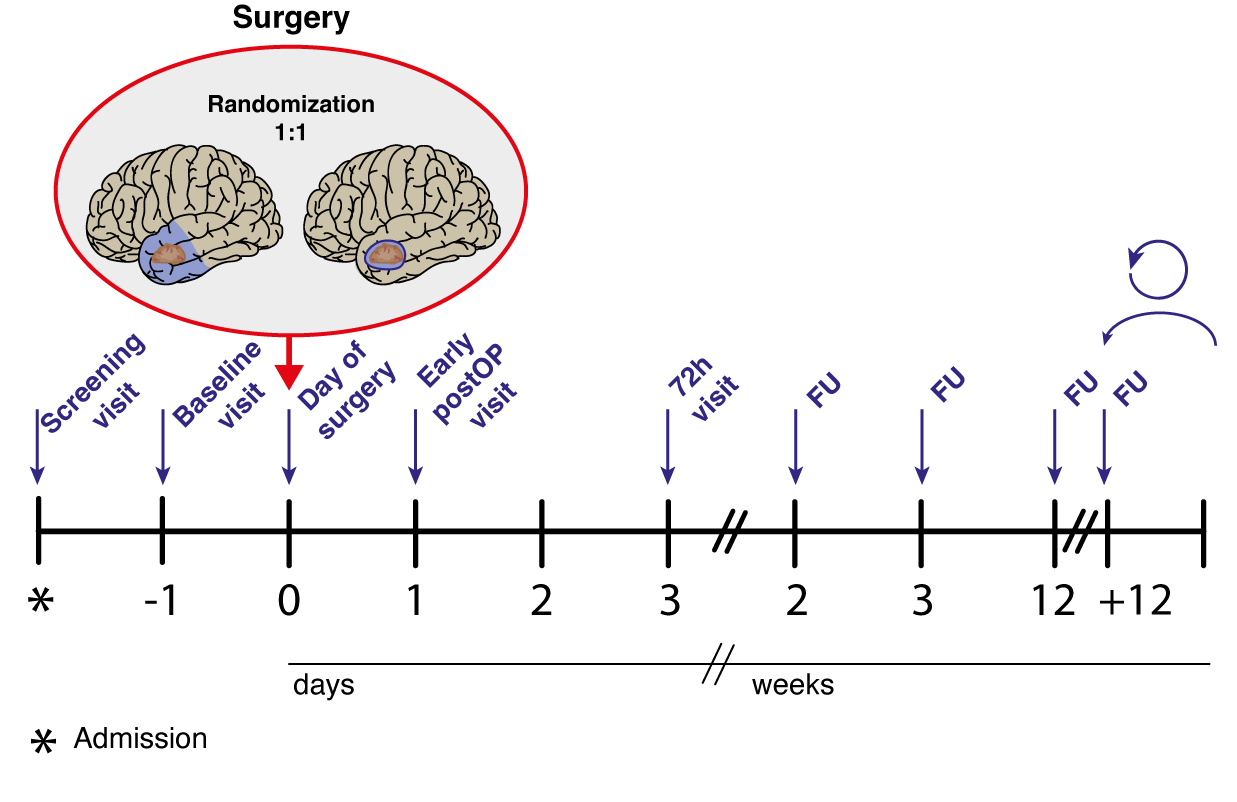
Trial design
The ATLAS-trial is a randomized, controlled phase III trial with 26 trials sites.
Inclusion criteria
- Suspected glioblastoma with contrast-enhancement in MRI
- Diffuse high-grade glioma in frozen section procedure, newly-diagnosed
- Tumor localization of the contrast-enhancing lesion on MRI in the temporal lobe: non-dominant side (right hemisphere in right-handed patients, or left-handed patients after testing for dominance): within 6.5 cm from the temporal pole OR; dominant side (left hemisphere in right-handed patients, all left-handed patients unless additional testing for dominance performed): within 4.0 cm from the temporal pole, as determined dorsally along the Sylvian fissure
- Macroscopic complete resection (no remaining contrast-enhancing tumoral lesion on early postoperative MRI) is achievable (decision of the treating neurosurgeon)
- In case further T1-contrast-enhancing and/or T2 and/or FLAIR lesions are detected beyond the resection margins (6.5 cm on the non-dominant side and 4.0 cm on the dominant side), these lesions are not attributed to the tumor (except perifocal edema) but to other conditions according to the local treating neurosurgeon
- ≥ 18 and < 75 years of age
- KPS ≥ 70%
- Estimated life expectancy of at least 6 months
- Written informed consent
- Cognitive state to understand the rationale and necessity of study therapy and procedures
- Patient compliance and geographic proximity that allow adequate follow-up examinations
- For patients with childbearing potential: negative serum pregnancy test (beta-HCG) at baseline visit, patient’s commitment to use an approved contraceptive method during the trial and for 3 months after (Pearl index < 1%)
- Adequate organ function that does not preclude alkylating chemotherapy and neurosurgical procedures, as described below:
- Adequate bone marrow reserve
- White blood cell (WBC) count ≥ 3000/µl
- Granulocyte count > 1500/µl
- Platelets ≥ 100000/µl
- Haemoglobin ≥ 10 g/dl
- Adequate liver function
- Bilirubin < 1.5 times above upper limit of normal range (ULN)
- Alanine transaminase (ALT/SGPT) and aspartate transaminase (AST/ALAT) < 3 times ULN
- Adequate renal function
- Creatinine < 1.5 times ULN
- Adequate bone marrow reserve
- Adequate blood clotting: PTT not exceeding the upper limit of normal range and INR < 1.5; in case of intake of anticoagulant medication or platelet function inhibitors, the coagulation analysis must show no detectable effect in specific blood tests (as described below) at the time of surgery, and discontinuation of the anticoagulant medication must be justifiable for at least 1 week postoperatively
- Direct acting oral anticoagulants (e.g., Rivaroxaban, Apixaban, Edoxaban, Dabigatran): aFXa-activity (anti-factor Xa) within the normal range (Rivaroxaban, Apixaban, Edoxaban), TT/TCT (thrombin clotting time) or ECT (ecarin clotting time) not exceeding the upper limit of normal range (Dabigatran) or verification of subtherapeutic drug levels (Apixaban, Edoxaban, Rivaroxaban, Dabigatran)
- Vitamin K antagonists (Coumarins): INR < 1.5
- Unfractionated heparin (UFH) and Argatroban: aPTT not exceeding the upper limit of normal range
- Fractionated heparin/low-molecular-weight heparin (e.g., Dalteparin, Enoxaparin), heparinoid (e.g., Fondaparinux, Danaparoid): aFXa-activity within the normal range
- Antiplatelet agents (Aspirin, Clopidogrel, Prasugrel, Ticagrelor): PFA (platelet function analyzer) test not exceeding the upper limit of normal range (Aspirin), whole blood aggregometry not below lower limits of normal range (Clopidogrel, Prasugrel, Ticagrelor)
Study protocol
The trial will randomize 178 patients over 3 years with a follow-up of 3 years after inclusion of the last patient. Assuming a prolongation of median survival time from 17 months to 27.5 months for the ATL approach and constant recruitment, this sample size provides a power of above 80% for the intended two-sided stratified log-rank test (level of 5%) to detect differences in OS between the arms.
The trial will be monitored by a Data Safety Monitoring Board. An interim safety analysis 6 months after recruitment of 1/3 of the patients will be performed to make sure that the postoperative clinical status is not significantly worse in the experimental (ATL) arm compared to the standard (GTR) arm. Safety will be objectified by the rate of patients with a postoperative modified ranking scale (mRS) score of 4-6. Particular attention will also be paid to periprocedural adverse events as measured by patient safety indicators and specific cranial surgery-related complications.
Accompanying scientific program
The resected anterior temporal lobe tissue provides the unique opportunity for cross-sectional analyses of tumor infiltration and microenvironment ranging from the central bulky tumor part to various degrees of the infiltration zone including areas without overt signs of infiltration on conventional MRI. We aim at creating a map of cellular components, functional states and network interactions covering vast parts of the infiltration zones of the tumor.
Recruitment
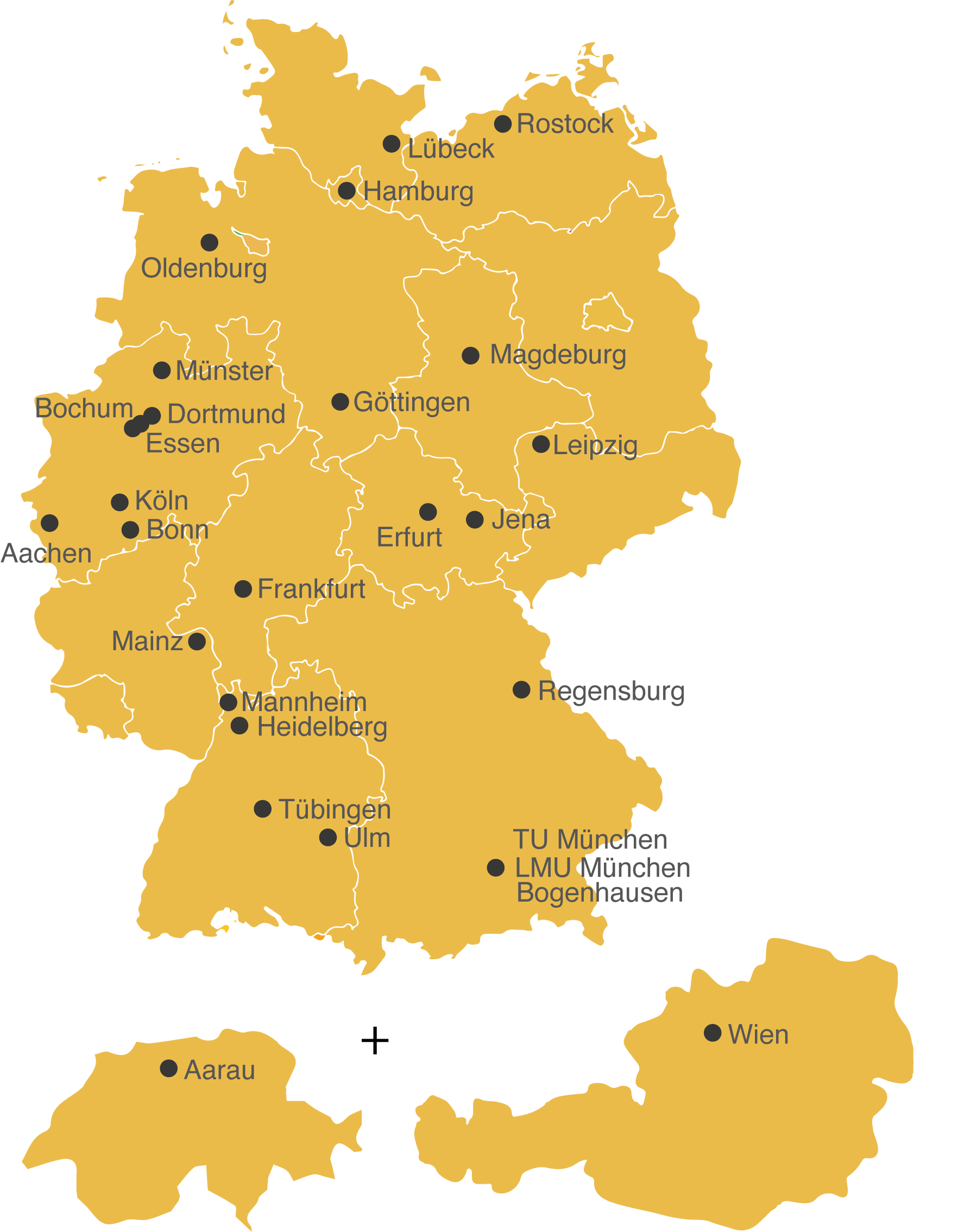
Initiation of Bonn trial site: Nov 2024
(Effective: 23 Feb 2025)
Funding

Trial registration
German Clinical Trials Register (DRKS): https://www.drks.de/search/de/trial/DRKS00035314