Cellular networks in glioblastoma
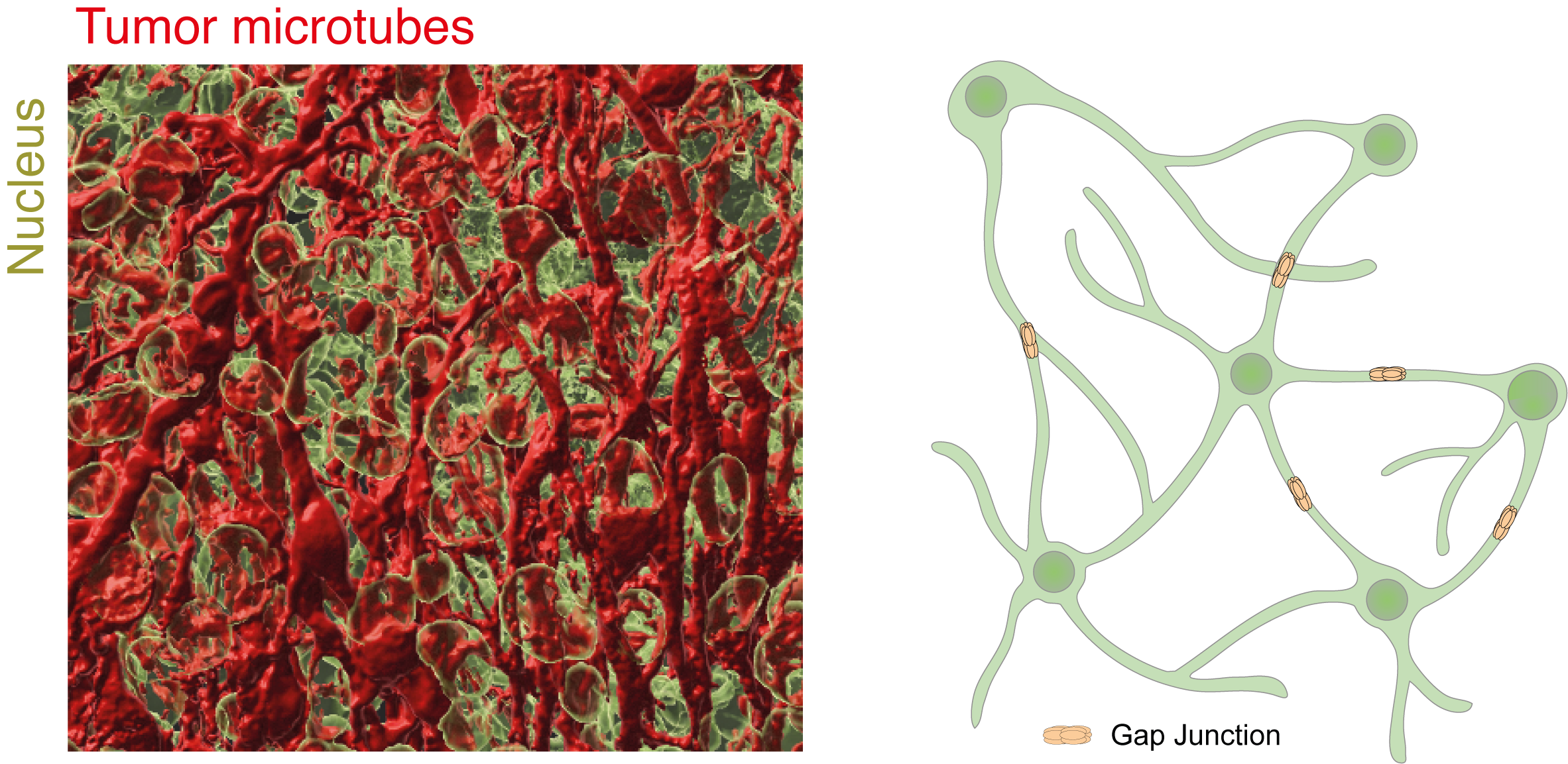
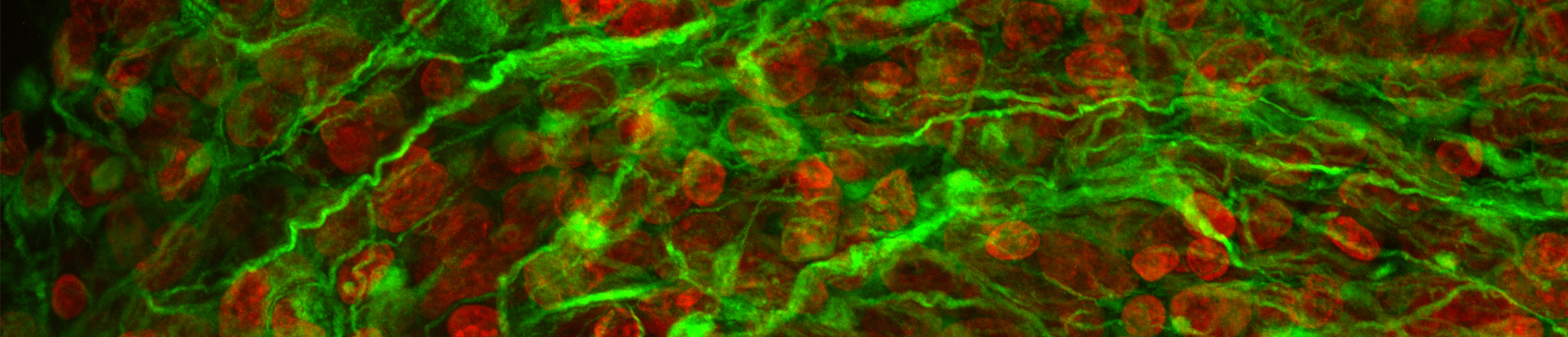
Tumor microtubes interconnect glioblastoma cells to a multicellular network
Glioblastoma is the most common malignant primary brain tumor in adults. Despite a multimodal therapeutic standard of care consisting of maximal-safe tumor resection followed by radio- and chemotherapy median life expectancy only reaches up to about 18 months. One of the main obstacles of more effective therapies is the tumor cells' capacity of forming ultra-long and highly dynamic membrane protrusions - so-called tumor microtubes - that integrate glioblastoma cells into a multicellular syncytial network and enable intercellular long distance communication.
Our experimental research is focused on deciphering the cellular mechanisms of tumor microtube formation and how tumor microtubes contribute to therapy resistance and micro-invasive capacity. We use a wide range of preclinical tumor models to mimic the human physiological in-vivo conditions including long-term cultures of glioblastoma cells in human neocortical brain slices and patient-derived tumor organoides. We perform comprehensive patient sample analysis (among them single-cell RNA sequencing and co-detection by indexing (CODEX) multiplexed imaging technology) and employ multi-modal imaging settings and computational analyses.
We currently have several projects aimed at exploring the mechanisms of tumor microtube outgrowth and delineating the local heterogeneity of cellular network connectivity in glioblastoma. Based on this knowledge, we continue to perform high-throughput screening for pharmacological compounds that disrupt tumor microtube-based network communication and intend to design clinical trials to transfer these findings from bench to bedside.
Bridging basic science into clinical reality
In a previous study, we have demonstrated that meclofenamate − a nonsteroidal anti-inflammatory drug with clinical use in the U.S. – causes morphological and functional breakdown within these fatal tumor networks. This breakdown was driven by the inhibition of tumor microtube outgrowth and reduction of cytosolic traffic from cell to cell via tumor microtubes. Based on these findings, we have initiated an investigator-initiated nation-wide phase I/II clinical trial (MecMeth) which evaluates the additional use of meclofenamate in recurrent glioblastoma.
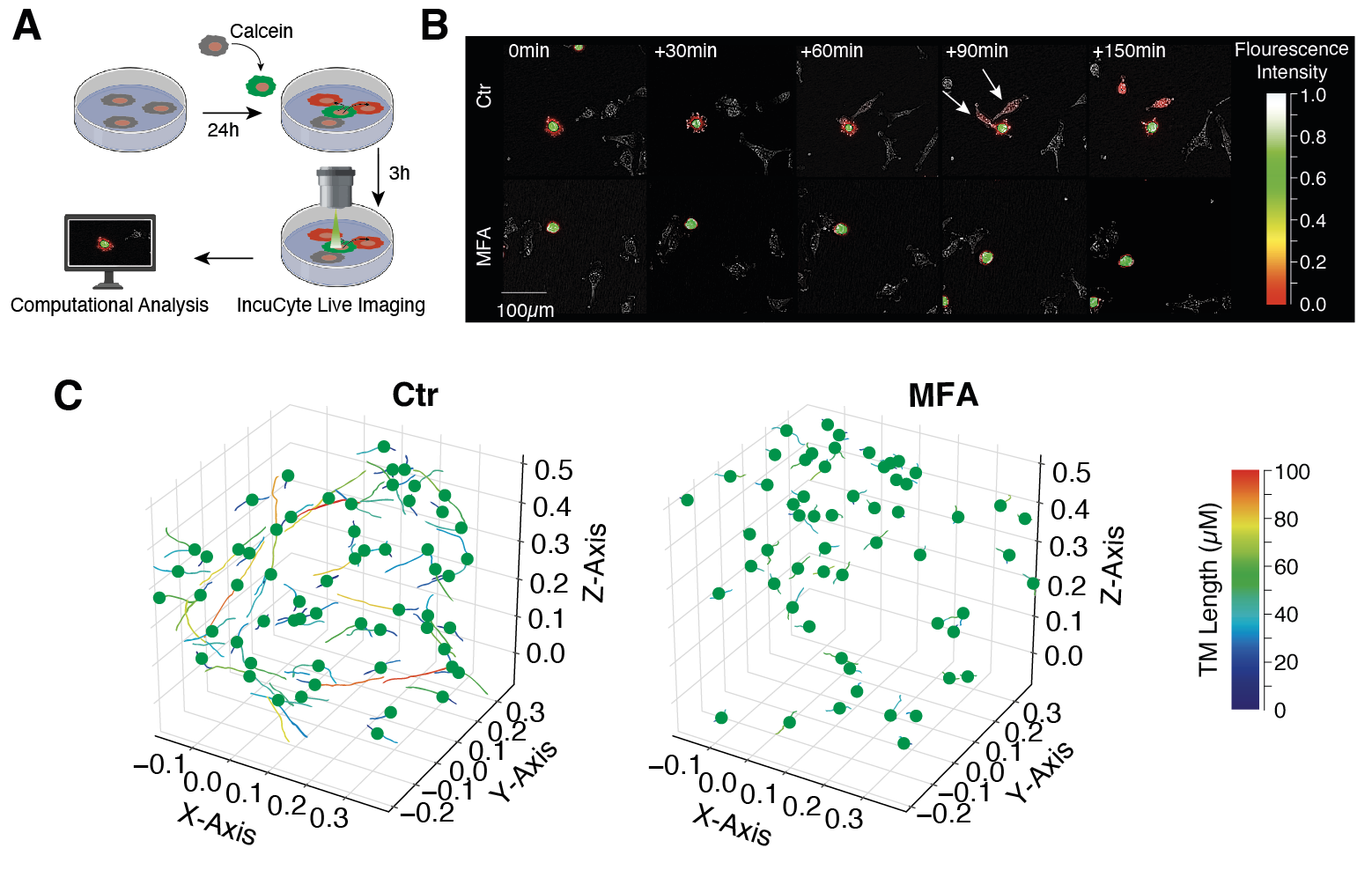
Meclofenamate (MFA) disrupts glioblastoma network connectivity on the functional and morphological level
(A) Graphical illustration of calcein dye transfer experiments to visualize intercellular cytosolic traffic. (B) MFA inhibits calcein transfer between calcein donor cells (green) and calcein receiver cells (red). (C) 3-dimensional reconstruction of the malignant network after injection of primary glioblastoma cells into human brain slice cultures. MFA destroys glioblastoma network morphology which is reflected by a significant reduction of tumor microtube length. Modified from Schneider et al., Neuro-Oncology, 2021
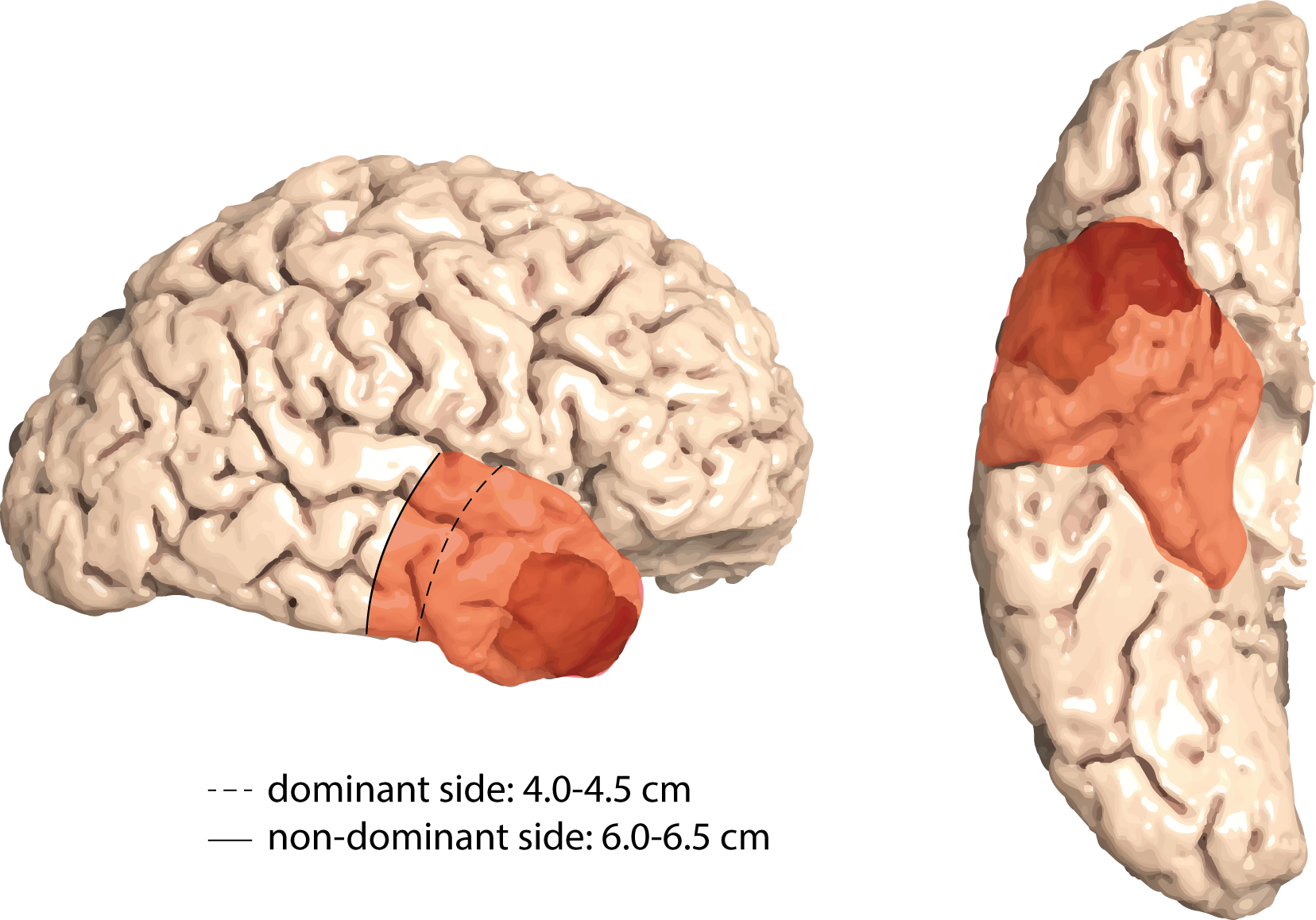
Anterior temporal lobectomy (ATL) as a paradigm for supramarginal resection
With the knowledge of cellular tumor networks that reach far beyond the macroscopically-visible tumor margins, the concept of supramarginal resection strategies has gained growing interest. Compared to a conventional gross-total resection (GTR), supramarginal approaches capture tumor networks beyond the tumor bulk margins and are supposed to provide superior long-term disease control. However, with regard to adjacent eloquent brain tissue and critical vasculature at risk, supramarginal resection regimes might be accompanied by a loss of neurological function therefore restricting supramarginal resections to a selected group of tumor localizations. Resection of the anterior temporal lobe - via the so-called anterior temporal lobectomy (ATL) - is a procedure commonly performed in temporal lobe epilepsy surgery and stands out as an anatomically highly standardized surgical approach. Therefore, translation of ATL from epilepsy surgery to the field of oncological neurosurgery enables to assess ATL as a paradigm for supramarginal resection in patients with temporal glioblastoma.
In a retrospective study we analyzed all patients that had been resected for temporal glioblastoma at the Neuro-Oncology Center of the University Hospital Bonn between 2012 and 2018. Our data suggest that supramarginal resection in terms of an ATL is accompanied by a significant survival benefit compared to a conventional gross-total resection (GTR) approach. Based on these single center data we designed a multicenter randomized clinical trial (ATLAS) which will evaluate ATL as a paradigm for supramarginal resection in temporal glioblastoma surgery.
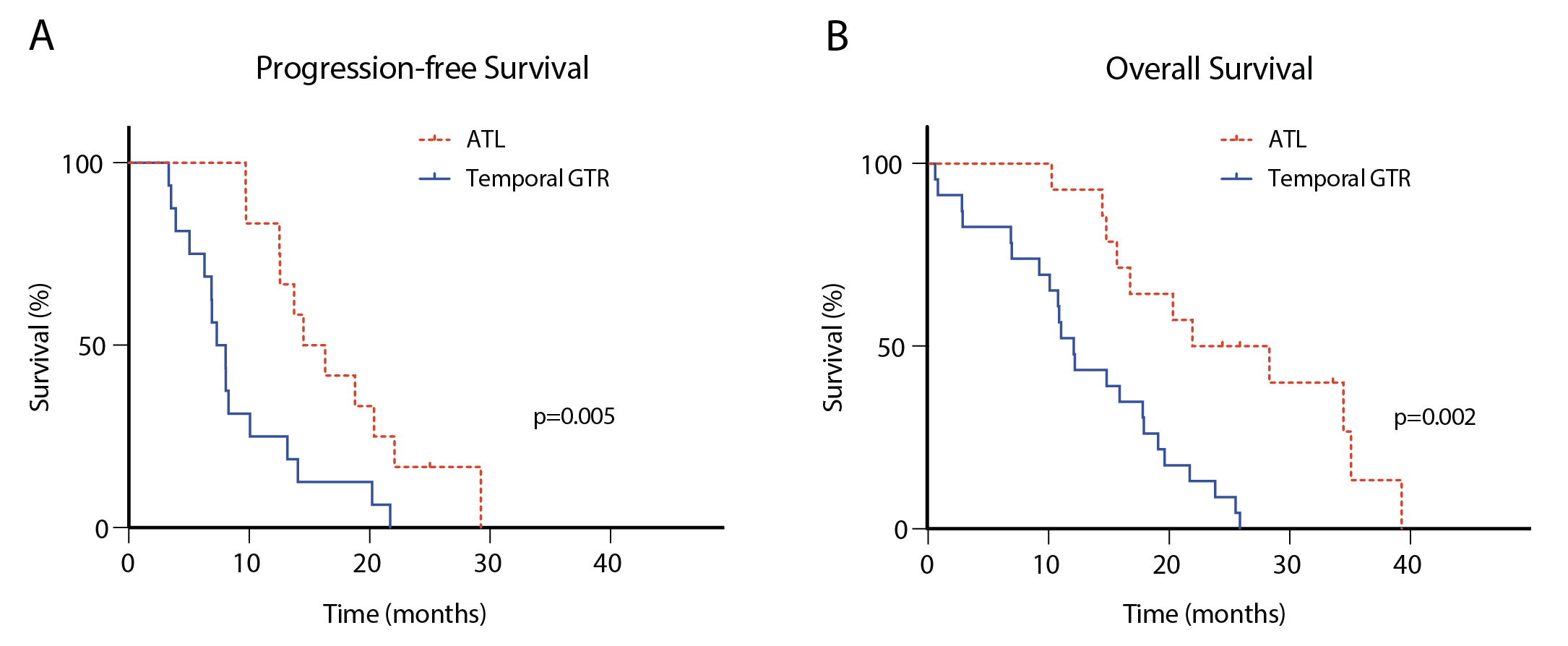
Anterior temporal lobectomy (ATL) shows superiority in temporal glioblastoma surgery
ATL is accompanied by prolonged progression-free survival (A) and overall survival (B) compared to a conventional gross-total resection (GTR) in patients with temporal glioblastoma. Modified from Schneider et al., Journal of Neuro-Oncology, 2019
Bi-modal therapeutic approach
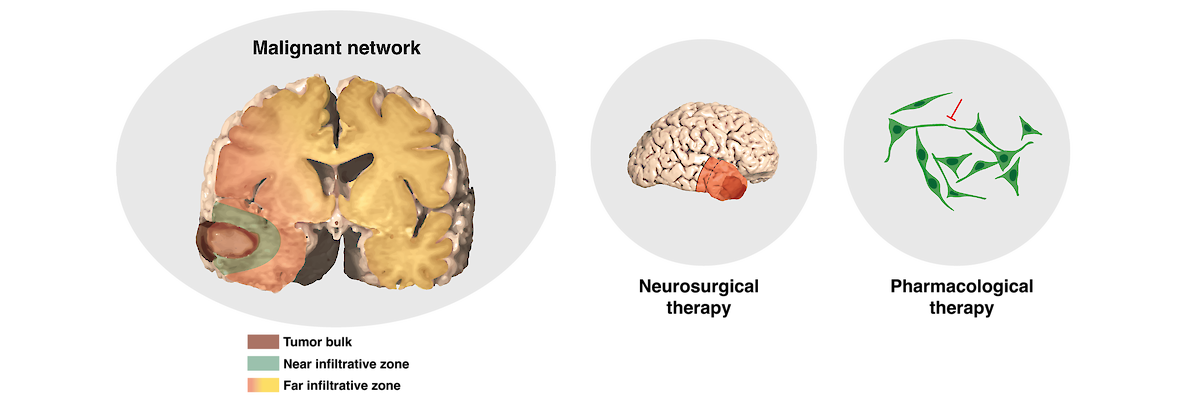
Our ultimate goal is to develop a therapeutic approach that will target glioblastoma's network connectivity in a bi-modal way:
1) Supramarginal resection - if feasible dependent on the tumor location - enables a high degree of network destruction by removal not only of the tumor bulk, but also the near infiltrative zone and adjacent areas of the far infiltrative zone.
2) Pharmacological therapy allows morphological and functional inhibition of the postoperatively remaining cellular networks of the far infiltrative tumor zone.
In view of the ATLAS-trial which will assess supramarginal resection in temporal lobe glioblastoma as well as the ongoing MecMeth-trial which evaluates Meclofenamate as a potential tumor microtube-targeted drug in patients with recurrent glioblastoma, this dual-modal therapeutic concept has yet reached the stage of clinical evaluation. Accompanying scientific work programs will gain insights into the heterogenous landscape as well as the morphological and transcriptional plasticity of tumor networks under conventional and novel therapeutic approaches. We hope that we will be able to answer in the near future whether this concept will finally succeed in enhancing the effectiveness of current standard of care.