Research
Germ cells propagate the genetic information from generation to generation. After fertilization, they give rise to the totipotent zygote. In the developing mammalian embryo, primordial germ cells (PGCs) are specified after induction by Bmp’s. These PGCs start to migrate and enter the genital ridge where they initiate sexual differentiation and eventually become oogonia or spermatogonia. PGCs express the pluripotency factors Oct3/4 and Nanog, but do not differentiate. This is due to the fact, that PGCs also express of Tfap2c, Prdm14 and Blimp1, which suppress somatic differentiation. Using germ cell specific deletion of Tfap2c we demonstrated, that Tfap2c is indispensable for this repression (Weber, et al., 2010). Germ cell tumors arise mostly from developmentally arrested PGCs. In the gonad, they can be detected as dormant precursor lesion, the germ cell neoplasia in situ (GCNIS). With puberty, GCNIS cells start to proliferate and develop into a seminoma, which remains undifferentiated, or an embryonal carcinoma, which displays features indicative of pluri- or totipotency. Of note, GCNIS and seminomas are highly similar to PGCs with regard to global gene expression and epigenetic pattern. We showed, that the PGC-specification genes Tfap2c and Blimp1 are expressed in CIS and seminoma but are downregulated in embryonal carcinoma (Pauls et al., 2005, Eckert et al., 2008). Our results suggest, that the cancer/testis antigen PRAME supports the pluripotency network and represses somatic and germ cell differentiation in seminomas (Nettersheim et al 2016a) (commented in Brit. Journal of Cancer). Using an interspecies transplantation approach, we demonstrated, that molecular crosstalk between tumor and stroma cells influences the fate of the tumor (Nettersheim et al., 2015). Further investigations revealed that epigenetic drugs (Romidespin and JQ1) have a profound effect on germ cell tumor cell lines suggesting that such drugs could add to the treatment regimen of patients suffering from e.g. cisplatin resistant germ cell tumors (Nettersheim et al. 2016b, Jostes et al. 2016). In a more recent study, we demonstrated, that Neddlyation contributes to Cisplatin resistance in germ cell tumors (Funke et. al, 2023).
Further to topic I., we are interested further stages of germ cell development, especially the spermiogenesis. Here, one of the last steps is the replacement of the histones by basic proteins, the protamines resulting in chromatin-hypercondensation. Using CRISPR/Cas9 gene editing in murine oocytes, we established a Protamine-2 deficient mouse strain. While male mice deficient for Protamine-2 are sterile, Prm-2 heterozygous animals are fertile while showing a reduction in Protamine-2 protein levels. We are able to demonstrate, that the lack of Protamine leads to DNA damage, triggering a ROS based cascade (Schneider et al. 2020). In addition, we do have now also models for deficiency of Protamine 1, where we see defective DNA-Protamination and, to our surprise also a defect in processing of Protamine 2 (Merges et al., 2023). In contrast to Protamine 1, which is expressed in a ready to use form, Protamine 2 comes in a precursor version, which during incorporation into Sperm DNA, gets processed (the 5' end is being cut off) to the so-called mature Protamine 2. In order to investigate the effects of mature Prm-2, we generated a mouse, deficient of the cleaved portion of Prm 2 and find, that removal of transition proteins is impaired, and a key histone variant is not properly removed (Arevalo et al., 2023). Having developed more Protamine deficient models, we anticipate to learn more about the complex molecular processes of DNA-hypercondensation in mammals.
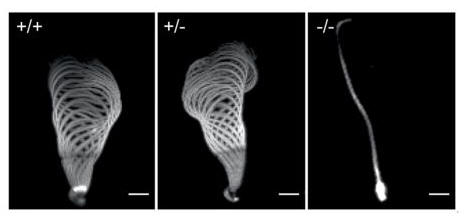
From Schneider et al., Scientific Reports, 2016.
Being interested in spermiogenesis per se, we got interested in genes, which are exclusively expressed during this important window of time in development. Sperm cells undergo a highly dynamic change of shape, round cytoplasma-rich cells become sleek and slender sperm cells. This change of shape is only begun to be understood and we hope to contribute a part to this story. We generated mouse models deficient in Profilins 3 and 4 which are supposed to interact with actin and actin dynamics. Interestingly, we found, that loss of Profilin 4 leads to a disturbance of the acrosome biogenesis, rendering the mice subfertile. We show, that loss of Profilin 3 leads to a deregulation of TRIM71, which impinges and derails protein levels important for the autophagic flux. Since parts of the autophagy machinery are re-used in spermiogenesis to help generating the acrosome, this cellular organelle is impaired in its function (Umer et al. 2021). Further, we published results of mouse lines being deficient for Actl7b (Merges et al., 2023) and Cylicin1 and Cylicin2 (Schneider, Kovacevic et al., 2023). We found, that sperm of humans harboring variants of CYLC1 and CYLC2 display analogous defects in sperm bauplan and are infertile as well.
Since our experiments require the establishment of genetically modified mice, our lab features a complete transgenic facility. Using homologous recombination in embryonic stem cells (ESCs), we have established several knock-out (Werling et al 2002, 2003) and knock-in (Jäger et al., 2004, Moenning et al., 2009, Holl et al., 2011, Haas et al., 2015) mouse lines. As early as 2014 we have established the production of genome edited mice technology using oocyte injection of the CRISPR-Cas9 system (Schneider et al., 2016). We further have the setup to derive ESCs, TSCs and also EG-lines (from PGCs). All these techniques are available to the general scientific community via the Core Facility "gene editing" integrated in the Bonn Technology Campus. The head of the Core Facility is still connected to the Schorle-Lab and participates in research related to germ cell biology, please see here for further details and also, if you would like to discuss own CRISPR-based murine projects.
References (see Publications page)

















