Research-Interests
Current lab members:
David Stahl, research associate
David Molitor, doctoral student
Verena Nowak, doctoral student
Research Interests
The main interest of my lab is how the tumor microenvironment (TME) influences tumor initiation, progression and therapy. The TME plays in all steps of tumorigenesis and immune escape has been postulated as a novel hallmark in cancer in 2011 by Hanahan and Weinberg. Knowledge about the TME in solid tumors recently has rapidly advanced, mainly because immunotherapies have become available targeting stromal cells and immune cells. In myeloproliferative disease, a hematopoetic stem cell neoplasm, a chronic immune dysregulation has been hypothesized as accompanying tumor evolution and is thought to even be the main target of current treatment strategies.
Research performed by my group and collaborators is in the following areas:
(i) TME in MYC induced liver tumors
(ii) Biomarker analysis in hepatobiliary, pancreatic tumors and hematolymphoid neoplasms
(iii) TME in myeloproliferative disease
Areas (i) and (ii) were addressed using a novel transgenic mouse model (c-myc/OVA) we devised as part of work of the Max Eder Program of the German Cancer, aim (ii) has been pursued using tissue microarrays and standard paraffin sections from human tumor samples. Aim (iii) is currently addressed using retrospective in silico-analysis using archived gene expression data sets (GSEs, gene expression omnibus GEO) accompanied by novel multiplex staining techniques in bone marrow sections in situ to allocate immune and stromal cells.
TME in MYC induced liver tumors
In order to test immunotherapeutic strategies within a natural tumor microenvironment we developed an autochthonous, c-myc induced murine tumor model (LAPtTA x c-myc/OVA) in which neo-antigen (chicken ovalbumin OVA specific immune responses can be studied easily (Ney et al. 2009). In this system, transferred OVA-specific CD8+ T-cells, although having excess to the tumor microenvironment are rendered tolerant and fail to mount an effective immune response. A triple combination approach using immunomodulatory antibodies, (CD137, OX40, PDL1) was able to activate endogenous CD8+ T-cells in vivo and synergized with adoptive T-cell therapy using activated OVA specific CD4+ and CD8+ T-cells to extend survival of these mice significantly (Morales et al. 2013). These results bear translational relevance as advanced hepatocellular carcinoma has a poor prognosis and immunmodulatory monoclonal antibodies are tested in clinical trials in cancer patients as monotherapies with encouraging results. Synergistic effects of several agents may also be exploited in other liver tumors, where the tumor microenvironment is a major hurdle to mount an effective immune response, such as metastatic liver disease.
A separate alley of research efforts has been to identify predictive markers in human carcinomas. We have identifed Fibroblast Growth Factor-1 (FGFR-1) as a potential novel therapeutic target in a small percentage of pancreatic carcinomas using tyrosine kinase receptor antagonists (von Maessenhausen et al. 2013) and a stem cell marker, Podocalyxin-like protein 1 (PODXL1)(Ney et al. 2007). Importantly, FGFR-1 amplified pancreatic cancer cells respond to FGFR-1 specific inhibitors in vitro. Furthermore, we have identified LSD-1 a histone demethylase to be expressed in a variety of hematolymphoid neoplasms (Niebel et al. 2014).
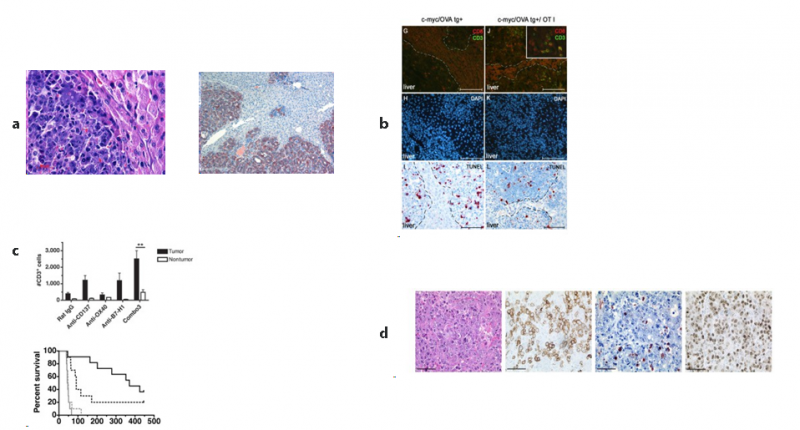
TME, liver tumors after successful immunotherapy, LAPtTA x c-MYC OVA transgenic mice. a. H&E morphology and expression of ovalbumin (neoantigen) in HCC tumor cells (IHC); b. Tracking of antigen specific OT1 TCR T-cells in vivo / apoptosis; c. immunotherapy (CD137, OX40, PDL1) using immunomodulatory antibodies plus preactivated CD4 and CD8 T-cells; d. T-cell infiltration and blasting before (top) and after immunotherapy (left to right: H&E, CD3, tunel, Ki-67)(Morales et al. 2013).
Biomarker analysis in hepatobiliary, pancreatic tumors and hematolymphoid neoplasms
A separate alley of research efforts has been to identify predictive markers in human carcinomas. We have identifed Fibroblast Growth Factor-1 (FGFR-1) as a potential novel therapeutic target in a small percentage of pancreatic carcinomas using tyrosine kinase receptor antagonists (von Maessenhausen et al. 2013) and a stem cell marker, Podocalyxin-like protein 1 (PODXL1)(Ney et al. 2007). Importantly, FGFR-1 amplified pancreatic cancer cells respond to FGFR-1 specific inhibitors in vitro (collaboration, Novartis). Furthermore, we have identified LSD-1 a histone demethylase to be expressed in a variety of hematolymphoid neoplasms (Niebel et al. 2014).

















